当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and biological evaluation of genistein‐O‐alkylamine derivatives as potential multifunctional anti‐Alzheimer agents
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-10-21 , DOI: 10.1111/cbdd.13414 Chen Hong 1 , Hui-yan Guo 2 , Shuai Chen 2 , Jian-wu Lv 2 , Xin Zhang 2 , Ya-cheng Yang 2 , Kang Huang 3 , Yi-juan Zhang 3 , Zhi-yong Tian 3 , Wen Luo 2 , Yi-ping Chen 4
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-10-21 , DOI: 10.1111/cbdd.13414 Chen Hong 1 , Hui-yan Guo 2 , Shuai Chen 2 , Jian-wu Lv 2 , Xin Zhang 2 , Ya-cheng Yang 2 , Kang Huang 3 , Yi-juan Zhang 3 , Zhi-yong Tian 3 , Wen Luo 2 , Yi-ping Chen 4
Affiliation
|
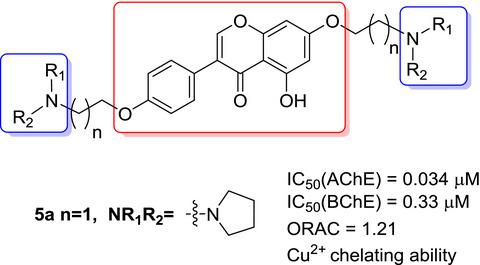
A series of genistein derivatives were synthesized and evaluated as multifunctional anti‐Alzheimer agents. The results showed that these derivatives had significant acetylcholinesterase (AChE) inhibitory activity; compound 5a exhibited the strongest inhibition to AChE with an IC50 value (0.034 μM) much lower than that of rivastigmine (6.53 μM). A Lineweaver–Burk plot and molecular modeling study showed that compound 5a targeted both the catalytic active site and the peripheral anionic site of AChE. These compounds also showed potent peroxy scavenging activity and metal‐chelating ability. The compounds did not show obvious effect on HepG2 and PC12 cell viability at the concentration of 100 μM. Therefore, these genistein derivatives can be utilized as multifunctional agents for the treatment of AD.
中文翻译:

染料木黄酮-O-烷基胺衍生物作为潜在的多功能抗阿尔茨海默病药物的合成和生物学评估
合成了一系列染料木黄酮衍生物,并被评估为多功能抗阿尔茨海默病药物。结果表明,这些衍生物具有明显的乙酰胆碱酯酶(AChE)抑制活性。化合物5a对AChE表现出最强的抑制作用,IC 50值(0.034μM)远低于rivastigmine(6.53μM)。Lineweaver-Burk图和分子建模研究表明,化合物5a同时针对AChE的催化活性位点和外围阴离子位点。这些化合物还显示出有效的过氧清除活性和金属螯合能力。在浓度为100μM时,这些化合物对HepG2和PC12细胞的活力没有明显的影响。因此,这些金雀异黄素衍生物可以用作治疗AD的多功能剂。
更新日期:2018-10-21
中文翻译:

染料木黄酮-O-烷基胺衍生物作为潜在的多功能抗阿尔茨海默病药物的合成和生物学评估
合成了一系列染料木黄酮衍生物,并被评估为多功能抗阿尔茨海默病药物。结果表明,这些衍生物具有明显的乙酰胆碱酯酶(AChE)抑制活性。化合物5a对AChE表现出最强的抑制作用,IC 50值(0.034μM)远低于rivastigmine(6.53μM)。Lineweaver-Burk图和分子建模研究表明,化合物5a同时针对AChE的催化活性位点和外围阴离子位点。这些化合物还显示出有效的过氧清除活性和金属螯合能力。在浓度为100μM时,这些化合物对HepG2和PC12细胞的活力没有明显的影响。因此,这些金雀异黄素衍生物可以用作治疗AD的多功能剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号