当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
QM/MM Study of the Reaction Mechanism of the Dehydratase Domain from Mammalian Fatty Acid Synthase
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-08 00:00:00 , DOI: 10.1021/acscatal.8b02616 Fabiola E. Medina 1 , Rui P. P. Neves 1 , Maria J. Ramos 1 , Pedro A. Fernandes 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-08 00:00:00 , DOI: 10.1021/acscatal.8b02616 Fabiola E. Medina 1 , Rui P. P. Neves 1 , Maria J. Ramos 1 , Pedro A. Fernandes 1
Affiliation
|
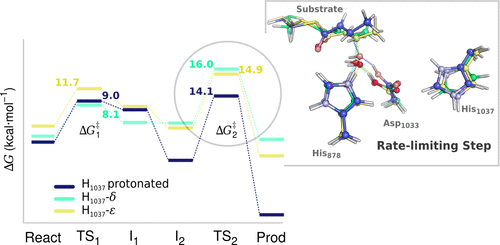
Dehydratase (DH) is a catalytic domain of the mammalian fatty acid synthase (mFAS), a multidomain enzyme with seven different active sites that work in tandem to carry out the biosynthesis of palmitic acid for de novo lipogenesis. DH catalyzes the dehydration of the β-hydroxyacyl to an α,β-unsaturated acyl intermediate. We have conducted hybrid QM/MM calculations to clarify the catalytic mechanism for the DH domain at the ONIOM(DFT/Amber) level of theory. The results have shown that the dehydration step occurs in two stages: (i) the His878-imidazole acts as a base deprotonating the Cα of the β-hydroxyacyl (HAC) substrate and (ii) the β-elimination of the β-hydroxyl of HAC proceeds with late protonation of the leaving hydroxide by the Asp1033-carboxylic group, forming a water molecule as a byproduct. The α-deprotonation depends on an oxyanion hole mechanism where the HAC’s α-carbonyl is anchored by two strong hydrogen bonds from the neighboring Gly888 and the intramolecular β-hydroxyl, positioning the Cα of HAC for deprotonation by His878. A positively charged His1037 improves the acidic character of Asp1033 and completes the catalytic triad in DH, because when His1037 is neutral the positively charged His878 behaves as the acid in the β-elimination step. We observe that the positively charged His1037 renders the β-elimination step more thermodynamically favorable (ΔrG of −15.9 kcal·mol–1). The β-elimination step exhibits a Gibbs energy barrier of 14.1 kcal·mol–1 and it is the rate-limiting step of the reaction (in agreement with the experimental barrier of ∼17 kcal·mol–1. Nevertheless, the rate-limiting step does not seem to be dependent on the protonation of His1037. Through evaluation of the electrostatic effect per residue on the rate-limiting step, we concluded also that the electrostatic contribution of the enzyme’s body does not seem significant, even though there are many positively and negatively charged residues close to the leaving β-hydroxyl group of HAC.
中文翻译:

QM / MM研究哺乳动物脂肪酸合酶脱水酶结构域的反应机理
脱水酶(DH)是哺乳动物脂肪酸合酶(mFAS)的催化结构域,mFAS是具有七个不同活性位点的多结构域酶,可协同工作以进行棕榈酸的生物合成,以进行从头开始的脂肪形成。DH催化β-羟基酰基脱水为α,β-不饱和酰基中间体。我们进行了混合QM / MM计算,以阐明ONIOM(DFT / Amber)理论水平上DH域的催化机理。结果表明,脱水步骤在两个阶段发生:(i)所述His878咪唑作为碱去质子化的C αβ-羟基酰基(HAC)底物的(a)和(ii)HAC的β-羟基的β-消除过程中,剩下的氢氧化物被Asp1033-羧基基团后期质子化,形成水分子副产物。α-去质子化取决于在HAC的α-羰基通过两个强的氢键从相邻Gly888和分子内β羟基锚定的C定位的氧离子洞机构α HAC的用于通过His878去质子化。带正电的His1037改善了Asp1033的酸性,并完成了DH中的催化三联体,因为当His1037为中性时,带正电的His878在β消除步骤中表现为酸。我们观察到带正电的His1037使β消除步骤在热力学上更有利(ΔrG为-15.9 kcal·mol –1。β-消除步骤显示出14.1 kcal·mol –1的吉布斯能垒,它是反应的限速步骤(与〜17 kcal·mol –1的实验势垒一致。步骤似乎并不依赖于His1037的质子化。通过评估限速步骤中每个残基的静电效应,我们还得出结论,尽管有很多积极的方面,酶体的静电作用似乎并不重要。和带负电荷的残基接近HAC的剩余β-羟基。
更新日期:2018-10-08
中文翻译:

QM / MM研究哺乳动物脂肪酸合酶脱水酶结构域的反应机理
脱水酶(DH)是哺乳动物脂肪酸合酶(mFAS)的催化结构域,mFAS是具有七个不同活性位点的多结构域酶,可协同工作以进行棕榈酸的生物合成,以进行从头开始的脂肪形成。DH催化β-羟基酰基脱水为α,β-不饱和酰基中间体。我们进行了混合QM / MM计算,以阐明ONIOM(DFT / Amber)理论水平上DH域的催化机理。结果表明,脱水步骤在两个阶段发生:(i)所述His878咪唑作为碱去质子化的C αβ-羟基酰基(HAC)底物的(a)和(ii)HAC的β-羟基的β-消除过程中,剩下的氢氧化物被Asp1033-羧基基团后期质子化,形成水分子副产物。α-去质子化取决于在HAC的α-羰基通过两个强的氢键从相邻Gly888和分子内β羟基锚定的C定位的氧离子洞机构α HAC的用于通过His878去质子化。带正电的His1037改善了Asp1033的酸性,并完成了DH中的催化三联体,因为当His1037为中性时,带正电的His878在β消除步骤中表现为酸。我们观察到带正电的His1037使β消除步骤在热力学上更有利(ΔrG为-15.9 kcal·mol –1。β-消除步骤显示出14.1 kcal·mol –1的吉布斯能垒,它是反应的限速步骤(与〜17 kcal·mol –1的实验势垒一致。步骤似乎并不依赖于His1037的质子化。通过评估限速步骤中每个残基的静电效应,我们还得出结论,尽管有很多积极的方面,酶体的静电作用似乎并不重要。和带负电荷的残基接近HAC的剩余β-羟基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号