当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A thermodynamic model for multivalency in 14-3-3 protein-protein interactions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-10-08 , DOI: 10.1021/jacs.8b09618 Loes M Stevers 1 , Pim J de Vink 1 , Christian Ottmann 1 , Jurriaan Huskens 2 , Luc Brunsveld 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-10-08 , DOI: 10.1021/jacs.8b09618 Loes M Stevers 1 , Pim J de Vink 1 , Christian Ottmann 1 , Jurriaan Huskens 2 , Luc Brunsveld 1
Affiliation
|
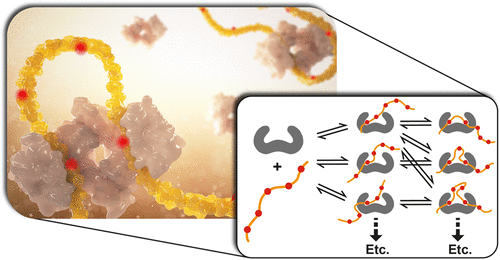
Protein–protein interactions (PPIs) are at the core of molecular control over cellular function. Multivalency in PPI formation, such as via proteins with multiple binding sites and different valencies, requires fundamental understanding to address correlated challenges in pathologies and drug development. Thermodynamic binding models are needed to provide frameworks for describing multivalent PPIs. We established a model based on ditopic host–guest systems featuring the effective molarity, a hallmark property of multivalency, as a prime parameter governing the intramolecular binding in divalent interactions. By way of illustration, we study the interaction of the bivalent 14-3-3 protein scaffold with both the nonavalent CFTR and the hexavalent LRRK2 proteins, determining the underlying thermodynamics and providing insights into the role of individual sites in the context of the multivalent platform. Fitting of binding data reveals enthalpy–entropy correlation in both systems. Simulations of speciations for the entire phosphorylated protein domains reveal that the CFTR protein preferably binds to 14-3-3 by combinations including the strongest binding site pS768, but that other binding sites take over when this site is eliminated, leading to only a minor decrease in total affinity for 14-3-3. For LRRK2, two binding sites dominate the complex formation with 14-3-3, but the distantly located pS1444 site also plays a role in complex formation. Thermodynamic modeling of these multivalent PPIs allowed analyzing and predicting the effects of individual sites regarding their modulation via, for example, (de)phosphorylation or small-molecule targeting. The results specifically bring forward the potential of PPI stabilization, as an entry for drug discovery for multivalent PPIs.
中文翻译:

14-3-3 蛋白质-蛋白质相互作用中多价的热力学模型
蛋白质-蛋白质相互作用 (PPI) 是细胞功能分子控制的核心。PPI 形成的多价性,例如通过具有多个结合位点和不同价态的蛋白质,需要基本的理解来解决病理学和药物开发中的相关挑战。需要热力学结合模型来提供描述多价 PPI 的框架。我们建立了一个基于双位主客体系统的模型,该模型具有有效摩尔浓度,多价的标志特性,作为控制二价相互作用中分子内结合的主要参数。作为说明,我们研究了二价 14-3-3 蛋白支架与九价 CFTR 和六价 LRRK2 蛋白的相互作用,确定潜在的热力学并深入了解单个位点在多价平台背景下的作用。结合数据的拟合揭示了两个系统中的焓-熵相关性。对整个磷酸化蛋白结构域的物种形成模拟表明,CFTR 蛋白优选通过包括最强结合位点 pS768 的组合与 14-3-3 结合,但当该位点被消除时,其他结合位点会接管,导致仅轻微下降总亲和力为 14-3-3。对于 LRRK2,两个结合位点在 14-3-3 的复合物形成中占主导地位,但距离较远的 pS1444 位点也在复合物形成中起作用。这些多价 PPI 的热力学建模允许分析和预测单个位点对其调制的影响,例如,(去)磷酸化或小分子靶向。结果特别提出了 PPI 稳定的潜力,作为多价 PPI 药物发现的一个入口。
更新日期:2018-10-08
中文翻译:

14-3-3 蛋白质-蛋白质相互作用中多价的热力学模型
蛋白质-蛋白质相互作用 (PPI) 是细胞功能分子控制的核心。PPI 形成的多价性,例如通过具有多个结合位点和不同价态的蛋白质,需要基本的理解来解决病理学和药物开发中的相关挑战。需要热力学结合模型来提供描述多价 PPI 的框架。我们建立了一个基于双位主客体系统的模型,该模型具有有效摩尔浓度,多价的标志特性,作为控制二价相互作用中分子内结合的主要参数。作为说明,我们研究了二价 14-3-3 蛋白支架与九价 CFTR 和六价 LRRK2 蛋白的相互作用,确定潜在的热力学并深入了解单个位点在多价平台背景下的作用。结合数据的拟合揭示了两个系统中的焓-熵相关性。对整个磷酸化蛋白结构域的物种形成模拟表明,CFTR 蛋白优选通过包括最强结合位点 pS768 的组合与 14-3-3 结合,但当该位点被消除时,其他结合位点会接管,导致仅轻微下降总亲和力为 14-3-3。对于 LRRK2,两个结合位点在 14-3-3 的复合物形成中占主导地位,但距离较远的 pS1444 位点也在复合物形成中起作用。这些多价 PPI 的热力学建模允许分析和预测单个位点对其调制的影响,例如,(去)磷酸化或小分子靶向。结果特别提出了 PPI 稳定的潜力,作为多价 PPI 药物发现的一个入口。











































 京公网安备 11010802027423号
京公网安备 11010802027423号