当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of Ozone and Kinetics of Its Decomposition in Aqueous Chloride Solutions
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-10-18 , DOI: 10.1021/acs.iecr.8b03371 Alexander V. Levanov 1 , Oksana Ya. Isaikina 1 , Ramiya B. Gasanova 1 , Valery V. Lunin 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-10-18 , DOI: 10.1021/acs.iecr.8b03371 Alexander V. Levanov 1 , Oksana Ya. Isaikina 1 , Ramiya B. Gasanova 1 , Valery V. Lunin 1
Affiliation
|
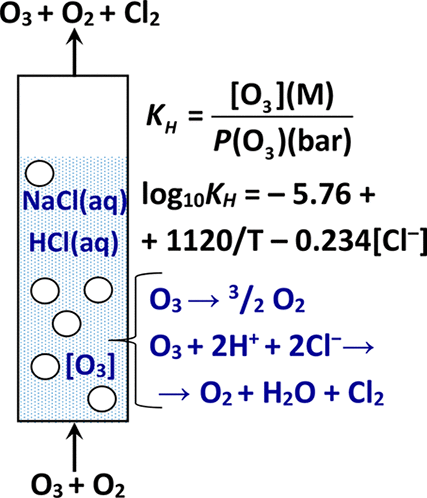
Solubility of ozone and kinetics of its decomposition in aqueous solutions of NaCl and NaCl + HCl have been investigated as functions of solution composition, electrolyte concentration, and temperature. A methodology has been developed, in which dissolved O3 concentration was measured by a direct spectrophotometric technique by absorbance at 260 nm, with ozone decomposition in the sample and the presence of other substances also absorbing light at 260 nm being taken into account. For solutions with concentrations of Cl– 0–2 M and H+ 0–0.5 M, the solubility coefficient (LO3) and Henry’s law constant of ozone (KH) have been determined in the temperature range 20–40 °C, as well as the kinetic order and apparent rate constants of O3 decomposition (at 20 ± 1 °C). Formulas have been obtained for calculating LO3 and KH for various values of temperature and electrolyte concentration. An equation is presented for computing the rate constant of ozone reaction with chloride ion in aqueous solution via the mechanism of oxygen atom transfer as a function of temperature and concentration of H+ ions.
中文翻译:

臭氧在氯化物水溶液中的溶解度及其分解动力学
已研究了臭氧的溶解度及其在NaCl和NaCl + HCl水溶液中的分解动力学,其与溶液组成,电解质浓度和温度的关系。已经开发了一种方法,其中通过直接分光光度法通过在260nm处的吸光度来测量溶解的O 3浓度,同时考虑了样品中的臭氧分解以及考虑到其他物质也吸收了260nm处的光。对于浓度为Cl – 0–2 M和H + 0–0.5 M的溶液,溶解度系数(L O3)和臭氧的亨利定律常数(K H)是在20–40°C的温度范围内确定的,还确定了O 3分解的动力学级数和表观速率常数(在20±1°C下)。已经获得了用于计算各种温度和电解质浓度的L O3和K H的公式。提出了一个方程,通过氧原子转移的机理,计算臭氧与水溶液中氯离子的反应速率常数与温度和H +离子浓度的关系。
更新日期:2018-10-19
中文翻译:

臭氧在氯化物水溶液中的溶解度及其分解动力学
已研究了臭氧的溶解度及其在NaCl和NaCl + HCl水溶液中的分解动力学,其与溶液组成,电解质浓度和温度的关系。已经开发了一种方法,其中通过直接分光光度法通过在260nm处的吸光度来测量溶解的O 3浓度,同时考虑了样品中的臭氧分解以及考虑到其他物质也吸收了260nm处的光。对于浓度为Cl – 0–2 M和H + 0–0.5 M的溶液,溶解度系数(L O3)和臭氧的亨利定律常数(K H)是在20–40°C的温度范围内确定的,还确定了O 3分解的动力学级数和表观速率常数(在20±1°C下)。已经获得了用于计算各种温度和电解质浓度的L O3和K H的公式。提出了一个方程,通过氧原子转移的机理,计算臭氧与水溶液中氯离子的反应速率常数与温度和H +离子浓度的关系。









































 京公网安备 11010802027423号
京公网安备 11010802027423号