当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Temperature and Pressure Effects on the Separation Efficiency and Desorption Kinetics in the TMA-CO2-H2O System
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-10-17 , DOI: 10.1021/acs.iecr.8b03926 Georgios Kolliopoulos 1 , Vladimiros G. Papangelakis 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-10-17 , DOI: 10.1021/acs.iecr.8b03926 Georgios Kolliopoulos 1 , Vladimiros G. Papangelakis 1
Affiliation
|
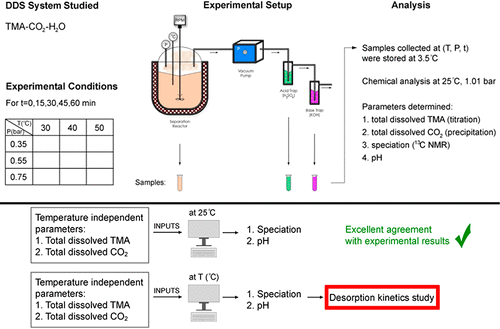
Draw solute separation from a draw solution is the main source of energy consumption in forward osmosis. The draw solute separation efficiency from a TMA-CO2-H2O (TMA = trimethylamine) draw solution and the decomposition kinetics of the latter were investigated in this study from 30 to 50 °C and 0.75 to 0.35 bar. The former proved stoichiometric and efficient with ∼100% draw solute removal after 60 min at 50 °C and 0.35 bar. The rate-determining step proved to be the physicochemical phenomenon of the desorption of the less volatile gas, TMA. It was found to be first order with respect to the TMA(aq) concentration. The combined effects of temperature and pressure on the k values proved that only the temperature has a significant effect. Finally, an Eyring–Arrhenius model was designed to estimate k values for the TMA desorption rate in the temperature and pressure range studied in this work.
中文翻译:

温度和压力对TMA-CO 2 -H 2 O系统分离效率和解吸动力学的影响
从抽提溶液中抽提溶质的分离是正向渗透过程中能耗的主要来源。在本研究中,在30至50°C和0.75至0.35 bar的条件下,研究了从TMA-CO 2 -H 2 O(TMA =三甲胺)汲取溶液中提取溶质的效率及其分解动力学。前者证明是化学计量的,并且在50°C和0.35 bar的压力下60分钟后能有效去除〜100%的溶质。决定速率的步骤被证明是挥发性较小的气体TMA解吸的物理化学现象。发现其相对于TMA(aq)浓度是一阶的。温度和压力对k的综合影响值证明只有温度才有显着影响。最后,设计了一个Eyring-Arrhenius模型来估计在这项工作中研究的温度和压力范围内TMA解吸速率的k值。
更新日期:2018-10-18
中文翻译:

温度和压力对TMA-CO 2 -H 2 O系统分离效率和解吸动力学的影响
从抽提溶液中抽提溶质的分离是正向渗透过程中能耗的主要来源。在本研究中,在30至50°C和0.75至0.35 bar的条件下,研究了从TMA-CO 2 -H 2 O(TMA =三甲胺)汲取溶液中提取溶质的效率及其分解动力学。前者证明是化学计量的,并且在50°C和0.35 bar的压力下60分钟后能有效去除〜100%的溶质。决定速率的步骤被证明是挥发性较小的气体TMA解吸的物理化学现象。发现其相对于TMA(aq)浓度是一阶的。温度和压力对k的综合影响值证明只有温度才有显着影响。最后,设计了一个Eyring-Arrhenius模型来估计在这项工作中研究的温度和压力范围内TMA解吸速率的k值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号