当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational-Switch Based Strategy Triggered by [18] π Heteroannulenes toward Reduction of Alpha Synuclein Oligomer Toxicity.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-10-19 , DOI: 10.1021/acschemneuro.8b00436 Ritobrita Chakraborty 1 , Sumit Sahoo 2 , Nyancy Halder 2 , Harapriya Rath 2 , Krishnananda Chattopadhyay 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-10-19 , DOI: 10.1021/acschemneuro.8b00436 Ritobrita Chakraborty 1 , Sumit Sahoo 2 , Nyancy Halder 2 , Harapriya Rath 2 , Krishnananda Chattopadhyay 1
Affiliation
|
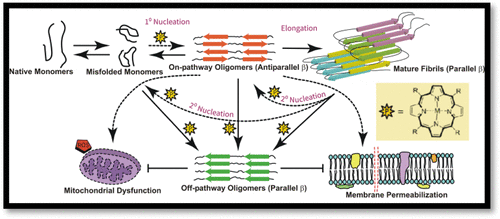
A water-soluble meso-carboxy aryl substituted [18] heteroannulene (porphyrin) and its Zn-complex have been found to be viable in targeting α-Syn aggregation at all its key microevents, namely, primary nucleation, fibril elongation, and secondary nucleation, by converting the highly heterogeneous and cytotoxic aggresome into a homogeneous population of minimally toxic off-pathway oligomers, that remained unexplored until recently. With the EC50 and dissociation constants in the low micromolar range, these heteroannulenes induce a switch in the secondary structure of toxic prefibrillar on-pathway oligomers of α-Syn, converting them into minimally toxic nonseeding off-pathway oligomers. The inhibition of the aggregation and the reduction of toxicity have been studied in vitro as well as inside neuroblastoma cells.
中文翻译:

由构象转换的策略,由[18]π杂环丁烯触发,以降低α突触核蛋白寡聚体的毒性。
已发现水溶性介孔羧基芳基取代的[18]杂亚环戊烯(卟啉)及其Zn-络合物在其所有关键的微事件(即初级成核,原纤维伸长和次级成核)上均可靶向α-Syn聚集。 ,通过将高度异质性和细胞毒性的聚集体转化为毒性最小的非通路寡聚体的同质群体,直到最近仍未开发。由于EC50和解离常数在低微摩尔范围内,这些杂环烯会诱导α-Syn的有毒原纤维在途中低聚物的二级结构发生转换,从而将它们转变为毒性最低的无种子非在途中低聚物。已经在体外以及在神经母细胞瘤细胞内部研究了聚集的抑制和毒性的降低。
更新日期:2018-10-08
中文翻译:

由构象转换的策略,由[18]π杂环丁烯触发,以降低α突触核蛋白寡聚体的毒性。
已发现水溶性介孔羧基芳基取代的[18]杂亚环戊烯(卟啉)及其Zn-络合物在其所有关键的微事件(即初级成核,原纤维伸长和次级成核)上均可靶向α-Syn聚集。 ,通过将高度异质性和细胞毒性的聚集体转化为毒性最小的非通路寡聚体的同质群体,直到最近仍未开发。由于EC50和解离常数在低微摩尔范围内,这些杂环烯会诱导α-Syn的有毒原纤维在途中低聚物的二级结构发生转换,从而将它们转变为毒性最低的无种子非在途中低聚物。已经在体外以及在神经母细胞瘤细胞内部研究了聚集的抑制和毒性的降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号