当前位置:
X-MOL 学术
›
Arab. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Validated spectrophotometric methods for simultaneous determination of oxytetracycline associated with diclofenac sodium or with piroxicam in veterinary pharmaceutical dosage form
Arabian Journal of Chemistry ( IF 5.3 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.arabjc.2018.09.007 Rúbia A. Sversut , James C. Vieira , Aline M. Rosa , Marcos S. do Amaral , Nájla M. Kassab , Hérida Regina Nunes Salgado
Arabian Journal of Chemistry ( IF 5.3 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.arabjc.2018.09.007 Rúbia A. Sversut , James C. Vieira , Aline M. Rosa , Marcos S. do Amaral , Nájla M. Kassab , Hérida Regina Nunes Salgado
|
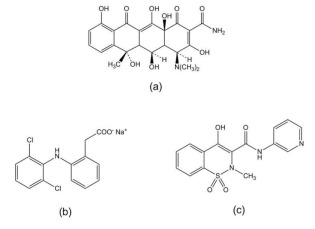
Abstract Two simple, precise, accurate and robust UV zero and first derivative orders spectrophometric methods have been developed and validated for the simultaneous determination of oxytetracycline (OTC) associated either with diclofenac sodium (DICLO) or with piroxicam (PIRO) in veterinary pharmaceuticals, without prior separation of the drugs. These proposed methods are also suitable for individual estimation of each drug in pharmaceutical products for human use. The first method is a zero order spectrophotometry for estimation of OTC at 360 nm in the association with DICLO. The second one employs a first derivative at 339 nm, using a zero-crossing technique to measure the OTC content in the association with PIRO. Both nonsteroidal anti-inflammatory drugs (DICLO and PIRO) were analyzed by first derivative spectrophometric at 298.5 nm (OTC zero-crossing point) in their respective associations. All measurements were carried out in acetronitrile:water (50:50, v/v; pH 2.5) and at room temperature (25 ± 2 °C). Analytical curves were linear (r > 0.9996) in the concentration range of 10.0–80.0 μg mL−1 for OTC, 1.5–25.0 μg mL−1 for DICLO and 2.5–30.0 μg mL−1 for PIRO. The limits of quantification were lower than 1.50 μg mL−1 and the mean of recoveries were within the acceptable limits of 98–102% for all three drugs. The developed methods can be successfully applied in the quality control routine for the simultaneous or individual determination of OTC, DICLO and PIRO in pharmaceutical dosage forms.
中文翻译:

经验证的分光光度法同时测定兽药剂型中与双氯芬酸钠或吡罗昔康相关的土霉素
摘要 已开发并验证了两种简单、精确、准确和稳健的 UV 零阶和一阶导数分光光度法,用于同时测定兽药中与双氯芬酸钠 (DICLO) 或吡罗昔康 (PIRO) 相关的土霉素 (OTC),无需预先分离药物。这些提议的方法也适用于对人类使用的药品中的每种药物进行单独估计。第一种方法是零级分光光度法,用于估计与 DICLO 相关的 360 nm 处的 OTC。第二个在 339 nm 处使用一阶导数,使用过零技术测量与 PIRO 相关的 OTC 含量。两种非甾体抗炎药(DICLO 和 PIRO)均在 298 处通过一阶导数分光光度法进行分析。5 nm(OTC 零交叉点)在它们各自的关联中。所有测量均在乙腈:水 (50:50, v/v; pH 2.5) 和室温 (25 ± 2 °C) 中进行。在 OTC 的 10.0-80.0 μg mL-1、DICLO 的 1.5-25.0 μg mL-1 和 PIRO 的 2.5-30.0 μg mL-1 的浓度范围内,分析曲线呈线性(r > 0.9996)。所有三种药物的定量限均低于 1.50 μg mL-1,回收率平均值在 98-102% 的可接受范围内。开发的方法可成功应用于质量控制程序,用于同时或单独测定药物剂型中的 OTC、DICLO 和 PIRO。在 OTC 的 10.0-80.0 μg mL-1、DICLO 的 1.5-25.0 μg mL-1 和 PIRO 的 2.5-30.0 μg mL-1 的浓度范围内,分析曲线呈线性(r > 0.9996)。所有三种药物的定量限均低于 1.50 μg mL-1,回收率平均值在 98-102% 的可接受范围内。开发的方法可成功应用于质量控制程序,用于同时或单独测定药物剂型中的 OTC、DICLO 和 PIRO。在 OTC 的 10.0-80.0 μg mL-1、DICLO 的 1.5-25.0 μg mL-1 和 PIRO 的 2.5-30.0 μg mL-1 的浓度范围内,分析曲线呈线性(r > 0.9996)。所有三种药物的定量限均低于 1.50 μg mL-1,回收率平均值在 98-102% 的可接受范围内。开发的方法可成功应用于质量控制程序,用于同时或单独测定药物剂型中的 OTC、DICLO 和 PIRO。
更新日期:2020-01-01
中文翻译:

经验证的分光光度法同时测定兽药剂型中与双氯芬酸钠或吡罗昔康相关的土霉素
摘要 已开发并验证了两种简单、精确、准确和稳健的 UV 零阶和一阶导数分光光度法,用于同时测定兽药中与双氯芬酸钠 (DICLO) 或吡罗昔康 (PIRO) 相关的土霉素 (OTC),无需预先分离药物。这些提议的方法也适用于对人类使用的药品中的每种药物进行单独估计。第一种方法是零级分光光度法,用于估计与 DICLO 相关的 360 nm 处的 OTC。第二个在 339 nm 处使用一阶导数,使用过零技术测量与 PIRO 相关的 OTC 含量。两种非甾体抗炎药(DICLO 和 PIRO)均在 298 处通过一阶导数分光光度法进行分析。5 nm(OTC 零交叉点)在它们各自的关联中。所有测量均在乙腈:水 (50:50, v/v; pH 2.5) 和室温 (25 ± 2 °C) 中进行。在 OTC 的 10.0-80.0 μg mL-1、DICLO 的 1.5-25.0 μg mL-1 和 PIRO 的 2.5-30.0 μg mL-1 的浓度范围内,分析曲线呈线性(r > 0.9996)。所有三种药物的定量限均低于 1.50 μg mL-1,回收率平均值在 98-102% 的可接受范围内。开发的方法可成功应用于质量控制程序,用于同时或单独测定药物剂型中的 OTC、DICLO 和 PIRO。在 OTC 的 10.0-80.0 μg mL-1、DICLO 的 1.5-25.0 μg mL-1 和 PIRO 的 2.5-30.0 μg mL-1 的浓度范围内,分析曲线呈线性(r > 0.9996)。所有三种药物的定量限均低于 1.50 μg mL-1,回收率平均值在 98-102% 的可接受范围内。开发的方法可成功应用于质量控制程序,用于同时或单独测定药物剂型中的 OTC、DICLO 和 PIRO。在 OTC 的 10.0-80.0 μg mL-1、DICLO 的 1.5-25.0 μg mL-1 和 PIRO 的 2.5-30.0 μg mL-1 的浓度范围内,分析曲线呈线性(r > 0.9996)。所有三种药物的定量限均低于 1.50 μg mL-1,回收率平均值在 98-102% 的可接受范围内。开发的方法可成功应用于质量控制程序,用于同时或单独测定药物剂型中的 OTC、DICLO 和 PIRO。











































 京公网安备 11010802027423号
京公网安备 11010802027423号