当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition of key enzymes in the inflammatory pathway by hybrid molecules of terpenes and synthetic drugs: In vitro and in silico studies.
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-10-30 , DOI: 10.1111/cbdd.13415 Cristina Theoduloz 1, 2 , Jans Alzate-Morales 3 , Felipe Jiménez-Aspee 2, 4, 5 , Maria Inés Isla 6 , María Rosa Alberto 6 , Mariano Walter Pertino 2, 7 , Guillermo Schmeda-Hirschmann 2, 7
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-10-30 , DOI: 10.1111/cbdd.13415 Cristina Theoduloz 1, 2 , Jans Alzate-Morales 3 , Felipe Jiménez-Aspee 2, 4, 5 , Maria Inés Isla 6 , María Rosa Alberto 6 , Mariano Walter Pertino 2, 7 , Guillermo Schmeda-Hirschmann 2, 7
Affiliation
|
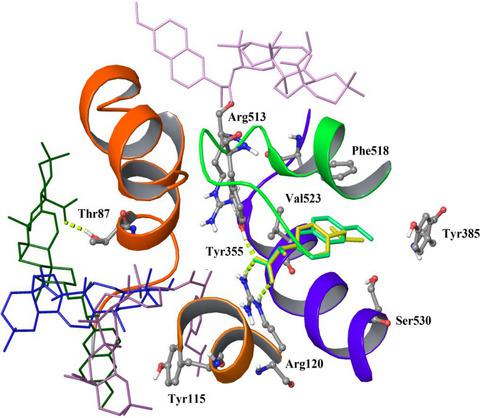
The aim of this work was to compare the anti-inflammatory activity of compounds prepared from terpenes and the synthetic drugs ibuprofen and naproxen. The anti-inflammatory activity of the hybrid compounds was compared with the activity of the parent compounds. This was accomplished using in vitro inhibition of lipoxygenases (LOX) and COX-2, and in silico docking studies in 15-LOX and COX-2. The synthesized hybrids showed an inhibition of COX-2 and LOX between 9.8%-57.4% and 0.0%-97.7%, respectively. None of the hybrids showed an improvement in the inhibitory effect toward these pro-inflammatory enzymes, compared to the parent terpenes and non-steroidal anti-inflammatory drugs. The docking studies allowed us to predict the potential binding modes of hybrids 6-15 within COX-2 and 15-LOX active sites. The relative affinity of the compounds inside the binding sites could be explained by forming non-covalent interactions with most important and known amino acids reported for those enzymes. A good correlation (r2 = 0.745) between docking energies and inhibition percentages against COX-2 was found. The high inhibition obtained for compound 10 against COX-2 was explained by hydrogen bond interactions at the enzyme binding site. New synthetic possibilities could be obtained from our in silico models, improving the potency of these hybrid compounds.
中文翻译:

萜类和合成药物的杂合分子对炎症途径中关键酶的抑制作用:体外和计算机研究。
这项工作的目的是比较由萜烯和合成药物布洛芬和萘普生制得的化合物的抗炎活性。将杂化化合物的抗炎活性与母体化合物的抗炎活性进行了比较。这是通过使用体外对脂氧合酶(LOX)和COX-2的抑制作用以及在15-LOX和COX-2中进行的计算机对接研究来实现的。合成的杂种显示出对COX-2和LOX的抑制分别在9.8%-57.4%和0.0%-97.7%之间。与亲本萜烯和非甾体类抗炎药相比,这些杂种均未显示出对这些促炎酶的抑制作用的改善。对接研究使我们能够预测COX-2和15-LOX活性位点内杂种6-15的潜在结合方式。化合物在结合位点内的相对亲和力可以通过与那些酶报道的最重要和已知的氨基酸形成非共价相互作用来解释。发现对接能与对COX-2的抑制百分比之间具有良好的相关性(r2 = 0.745)。化合物10对COX-2的高度抑制作用可以通过酶结合位点的氢键相互作用来解释。可以从我们的计算机模型中获得新的合成可能性,从而提高这些杂化化合物的效力。化合物10对COX-2的高度抑制作用是通过酶结合位点上的氢键相互作用来解释的。可以从我们的计算机模型中获得新的合成可能性,从而提高这些杂化化合物的效力。化合物10对COX-2的高度抑制作用是通过酶结合位点上的氢键相互作用来解释的。可以从我们的计算机模型中获得新的合成可能性,从而提高这些杂化化合物的效力。
更新日期:2018-10-30
中文翻译:

萜类和合成药物的杂合分子对炎症途径中关键酶的抑制作用:体外和计算机研究。
这项工作的目的是比较由萜烯和合成药物布洛芬和萘普生制得的化合物的抗炎活性。将杂化化合物的抗炎活性与母体化合物的抗炎活性进行了比较。这是通过使用体外对脂氧合酶(LOX)和COX-2的抑制作用以及在15-LOX和COX-2中进行的计算机对接研究来实现的。合成的杂种显示出对COX-2和LOX的抑制分别在9.8%-57.4%和0.0%-97.7%之间。与亲本萜烯和非甾体类抗炎药相比,这些杂种均未显示出对这些促炎酶的抑制作用的改善。对接研究使我们能够预测COX-2和15-LOX活性位点内杂种6-15的潜在结合方式。化合物在结合位点内的相对亲和力可以通过与那些酶报道的最重要和已知的氨基酸形成非共价相互作用来解释。发现对接能与对COX-2的抑制百分比之间具有良好的相关性(r2 = 0.745)。化合物10对COX-2的高度抑制作用可以通过酶结合位点的氢键相互作用来解释。可以从我们的计算机模型中获得新的合成可能性,从而提高这些杂化化合物的效力。化合物10对COX-2的高度抑制作用是通过酶结合位点上的氢键相互作用来解释的。可以从我们的计算机模型中获得新的合成可能性,从而提高这些杂化化合物的效力。化合物10对COX-2的高度抑制作用是通过酶结合位点上的氢键相互作用来解释的。可以从我们的计算机模型中获得新的合成可能性,从而提高这些杂化化合物的效力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号