当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical oxidative [4 + 2] annulation of tertiary anilines and alkenes for the synthesis of tetrahydroquinolines
Green Chemistry ( IF 9.3 ) Pub Date : 2018-10-08 , DOI: 10.1039/c8gc02463d Pengfei Huang 1, 2, 3, 4, 5 , Pan Wang 1, 2, 3, 4, 5 , Shengchun Wang 1, 2, 3, 4, 5 , Shan Tang 1, 2, 3, 4, 5 , Aiwen Lei 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2018-10-08 , DOI: 10.1039/c8gc02463d Pengfei Huang 1, 2, 3, 4, 5 , Pan Wang 1, 2, 3, 4, 5 , Shengchun Wang 1, 2, 3, 4, 5 , Shan Tang 1, 2, 3, 4, 5 , Aiwen Lei 1, 2, 3, 4, 5
Affiliation
|
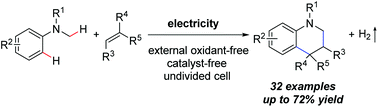
Heterocyclic compounds, especially nitrogen heterocycles, are one of the most important classes of compounds in the pharmaceutical and agrochemical industries. The oxidative [4 + 2] annulation reaction provides a powerful tool for the rapid construction of six-membered heterocycles. Herein, we report a metal- and external oxidant-free method for the uniform synthesis of tetrahydroquinolines through the electrochemical oxidative [4 + 2] annulation of tertiary anilines and alkenes. In this strategy, one partner loses only two hydrogen atoms while another partner reduces one degree of unsaturation, accompanied by the generation of hydrogen. Under the conditions of an undivided cell and room temperature, a series of tetrahydroquinoline derivatives were prepared with good yields.
中文翻译:

叔苯胺和烯烃的电化学氧化[4 + 2]环合成四氢喹啉
杂环化合物,特别是氮杂环,是制药和农业化学工业中最重要的化合物类别之一。氧化[4 + 2]环化反应为六元杂环的快速构建提供了强大的工具。在这里,我们报告了通过叔苯胺和烯烃的电化学氧化[4 + 2]环化法均匀合成四氢喹啉的无金属和外部氧化剂的方法。在这种策略中,一个配偶体仅损失两个氢原子,而另一个配偶体则减少了一个不饱和度,并伴随着氢的产生。在细胞未分裂且室温下的条件下,以高收率制备了一系列四氢喹啉衍生物。
更新日期:2018-10-30
中文翻译:

叔苯胺和烯烃的电化学氧化[4 + 2]环合成四氢喹啉
杂环化合物,特别是氮杂环,是制药和农业化学工业中最重要的化合物类别之一。氧化[4 + 2]环化反应为六元杂环的快速构建提供了强大的工具。在这里,我们报告了通过叔苯胺和烯烃的电化学氧化[4 + 2]环化法均匀合成四氢喹啉的无金属和外部氧化剂的方法。在这种策略中,一个配偶体仅损失两个氢原子,而另一个配偶体则减少了一个不饱和度,并伴随着氢的产生。在细胞未分裂且室温下的条件下,以高收率制备了一系列四氢喹啉衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号