Theranostics ( IF 12.4 ) Pub Date : 2018-10-06 , DOI: 10.7150/thno.26585 Sarah M Cheal 1, 2 , Hong Xu 3 , Hong-Fen Guo 3 , Mitesh Patel 2 , Blesida Punzalan 1, 2 , Edward K Fung 4 , Sang-Gyu Lee 4 , Meghan Bell 2 , Manisha Singh 1 , Achim A Jungbluth 5 , Pat B Zanzonico 4 , Alessandra Piersigilli 6 , Steven M Larson 1, 2 , Nai-Kong V Cheung 1, 3
|
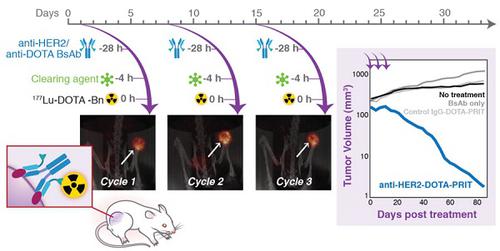
In recent reports, we have shown that optimized pretargeted radioimmunotherapy (PRIT) based on molecularly engineered antibody conjugates and 177Lu-DOTA chelate (DOTA-PRIT) can be used to cure mice bearing human solid tumor xenografts using antitumor antibodies to minimally internalizing membrane antigens, GPA33 (colon) and GD2 (neuroblastoma). However, many solid tumor membrane antigens are internalized after antibody binding and it is generally believed that internalizing tumor membrane antigens are not suitable targets for PRIT. In this study, we tested the hypothesis that DOTA-PRIT can be performed successfully to target HER2, an internalizing membrane antigen widely expressed in breast, ovarian, and gastroesophageal junction cancers.
Methods: DOTA-PRIT was carried out in athymic nude mice bearing BT-474 xenografts, a HER2-expressing human breast cancer, using a three-step dosing regimen consisting of sequential intravenous administrations of: 1) a bispecific IgG-scFv (210 kD) format (BsAb) carrying the IgG sequence of the anti-HER2 antibody trastuzumab and the scFv “C825” with high-affinity, hapten-binding antibody for Bn-DOTA (metal) (BsAb: anti-HER2-C825), 2) a 500 kD dextran-based clearing agent, followed by 3) 177Lu-DOTA-Bn. At the time of treatment, athymic nude mice bearing established subcutaneous BT-474 tumors (medium- and smaller-sized tumors with tumor volumes of 209 ± 101 mm3 and ranging from palpable to 30 mm3, respectively), were studied along with controls. We studied single- and multi-dose regimens. For groups receiving fractionated treatment, we verified quantitative tumor targeting during each treatment cycle using non-invasive imaging with single-photon emission computed tomography/computed tomography (SPECT/CT).
Results: We achieved high therapeutic indices (TI, the ratio of radiation-absorbed dose in tumor to radiation-absorbed dose to critical organs, such as bone marrow) for targeting in blood (TI = 28) and kidney (TI = 7), while delivering average radiation-absorbed doses of 39.9 cGy/MBq to tumor. Based on dosimetry estimates, we implemented a curative fractionated therapeutic regimen for medium-sized tumors that would deliver approximately 70 Gy to tumors, which required treatment with a total of 167 MBq 177Lu-DOTA-Bn/mouse (estimated absorbed tumor dose: 66 Gy). This regimen was well tolerated and achieved 100% complete responses (CRs; defined herein as tumor volume equal to or smaller than 4.2 mm3), including 62.5% histologic cure (5/8) and 37.5% microscopic residual disease (3/8) at 85 days (d). Treatment controls showed tumor progression to 207 ± 201% of pre-treatment volume at 85 d and no CRs. Finally, we show that treatment with this curative 177Lu regimen leads to a very low incidence of histopathologic abnormalities in critical organs such as bone marrow and kidney among survivors compared with non-treated controls.
Conclusion: Contrary to popular belief, we demonstrate that DOTA-PRIT can be successfully adapted to an internalizing antigen-antibody system such as HER2, with sufficient TIs and absorbed tumor doses to achieve a high probability of cures of established human breast cancer xenografts while sparing critical organs of significant radiotoxicity.
Keywords: multistep targeting, bispecific antibodies, HER2, radioimmunotherapy, pretargeting, lutetium-177
中文翻译:

在小鼠中人肿瘤异种移植中内化实体瘤抗原的肿瘤学预靶向放射免疫疗法:HER2阳性乳腺癌的治疗性治疗。
在最近的报道中,我们表明基于分子工程抗体偶联物和177 Lu-DOTA螯合物(DOTA-PRIT)的优化的预靶向放射免疫疗法(PRIT)可用于治疗携带人实体瘤异种移植物的小鼠,使用抗肿瘤抗体以最小化内在化膜抗原,GPA33(冒号)和GD2(神经母细胞瘤)。然而,许多实体肿瘤膜抗原在抗体结合后被内在化,并且通常认为内在化肿瘤膜抗原不是PRIT的合适靶标。在这项研究中,我们测试了DOTA-PRIT可以成功靶向HER2的假设,HER2是在乳腺癌,卵巢癌和胃食管连接癌中广泛表达的内在化膜抗原。
方法: DOTA-PRIT是在带有BT-474异种移植物(表达HER2的人类乳腺癌)的无胸腺裸鼠中进行的,采用三步给药方案,包括依次静脉内给药:1)双特异性IgG-scFv(210 kD )格式(BsAb),带有抗HER2抗体曲妥珠单抗的IgG序列和scFv“ C825”,具有针对Bn-DOTA(金属)的高亲和力,半抗原结合抗体(BsAb:抗HER2-C825),2)一种500 kD的基于葡聚糖的清洁剂,然后是3)177 Lu-DOTA-Bn。在治疗时,无胸腺裸鼠患有已确立的皮下BT-474肿瘤(中小型肿瘤,肿瘤体积为209±101 mm 3,可触及的范围为30 mm 3,分别与对照进行了研究。我们研究了单剂量和多剂量方案。对于接受分级治疗的组,我们使用单光子发射计算机断层扫描/计算机断层扫描(SPECT / CT)的非侵入性成像验证了每个治疗周期中的定量肿瘤靶向性。
结果:针对血液(TI = 28)和肾脏(TI = 7)的靶向性,我们获得了很高的治疗指数(TI,肿瘤中的辐射吸收剂量与关键器官(例如骨髓)的辐射吸收剂量之比),同时向肿瘤提供39.9 cGy / MBq的平均辐射吸收剂量。根据剂量学估算,我们对中型肿瘤实施了治疗性分级治疗方案,该方案可向肿瘤输送约70 Gy的剂量,每只小鼠总共需要167 MBq 177 Lu-DOTA-Bn(估计吸收的肿瘤剂量:66)进行治疗Gy)。该方案耐受性良好,可实现100%完全缓解(CR;在本文中定义为等于或小于4.2 mm 3的肿瘤体积),包括在85天时(d)的62.5%的组织学治愈率(5/8)和37.5%的微观残留疾病(3/8)。治疗对照组在85 d时肿瘤进展至治疗前体积的207±201%,无CR。最后,我们显示,与未治疗的对照组相比,这种治疗性177 Lu方案的治疗导致幸存者中关键器官如骨髓和肾脏的组织病理学异常发生率非常低。
结论:与普遍的看法相反,我们证明DOTA-PRIT可以成功地适应内在化的抗原-抗体系统(例如HER2),具有足够的TI和吸收的肿瘤剂量,从而在保留人类乳腺癌异种移植物的同时实现高治愈率重大放射毒性的关键器官。
关键词:多步靶向,双特异性抗体,HER2,放射免疫治疗,预靶向,177











































 京公网安备 11010802027423号
京公网安备 11010802027423号