当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual functions of ARP101 in targeting membrane type-1 matrix metalloproteinase: Impact on U87 glioblastoma cell invasion and autophagy signaling.
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-10-30 , DOI: 10.1111/cbdd.13410 Michel Desjarlais 1 , Borhane Annabi 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-10-30 , DOI: 10.1111/cbdd.13410 Michel Desjarlais 1 , Borhane Annabi 1
Affiliation
|
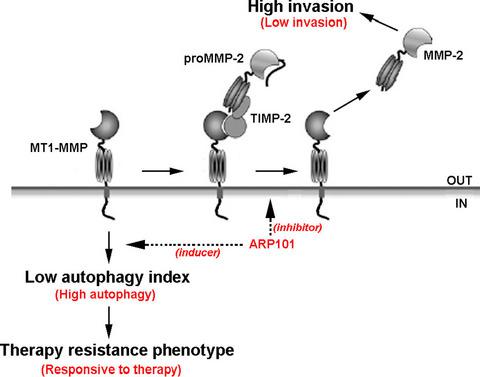
Membrane type-1 matrix metalloproteinase (MT1-MMP) possesses both extracellular proteolytic and intracellular signal-transducing functions in tumorigenesis. An imbalance in MT1-MMP expression and/or function triggers a metastatic, invasive, and therapy resistance phenotype. MT1-MMP is involved in extracellular matrix (ECM) proteolysis, activation of latent MMPs, as well as in autophagy signaling in human hepatoma and glioblastoma cells. A low autophagy index in tumorigenesis has been inferred by recent studies where autophagic capacity was decreased during tumor progression. Here, we establish ARP101 as a dual-function small-molecule inhibitor against MT1-MMP ECM hydrolysis and autophagy signal-transducing functions in a model of grade IV glioblastoma cells. ARP101 inhibited concanavalin-A-mediated proMMP-2 activation into MMP-2, as well as MT1-MMP auto-proteolytic processing. When overexpressing recombinant Wt MT1-MMP, ARP101 inhibited proMMP-2 activation and triggered the formation of MT1-MMP oligomers that required trafficking to the plasma membrane. ARP101 further induced cell autophagy as reflected by increased formation of acidic vacuole organelles, LC3 puncta, and autophagy-related protein ATG9 transcription. These were all significantly reversed upon siRNA-mediated gene silencing of MT1-MMP. ARP101 can thus concomitantly inhibit MT1-MMP extracellular catalytic function and exploit its intracellular transducing signal function to trigger autophagy-mediated cell death in U87 glioblastoma cancer cells.
中文翻译:

ARP101靶向膜1型基质金属蛋白酶的双重功能:对U87胶质母细胞瘤细胞侵袭和自噬信号的影响。
膜1型基质金属蛋白酶(MT1-MMP)在肿瘤发生中同时具有细胞外蛋白水解和细胞内信号转导功能。MT1-MMP表达和/或功能失衡会触发转移性,侵袭性和治疗耐药性表型。MT1-MMP参与细胞外基质(ECM)的蛋白水解,潜在MMP的激活以及人类肝癌和胶质母细胞瘤细胞的自噬信号传导。最近的研究推断,在肿瘤发生过程中自噬指数低,其中自噬能力在肿瘤进展过程中降低。在这里,我们建立ARP101作为双功能小分子抑制剂,针对IV级胶质母细胞瘤细胞模型中的MT1-MMP ECM水解和自噬信号转导功能。ARP101抑制伴刀豆球蛋白A介导的proMMP-2活化为MMP-2,以及MT1-MMP自动蛋白水解处理。当过表达重组Wt MT1-MMP时,ARP101抑制proMMP-2激活并触发MT1-MMP寡聚体的形成,从而需要转运至质膜。ARP101进一步诱导了细胞自噬,这反映为酸性液泡细胞器,LC3泪点和自噬相关蛋白ATG9转录的形成增加。当siRNA介导的MT1-MMP基因沉默时,所有这些都被显着逆转。因此,ARP101可以同时抑制MT1-MMP的细胞外催化功能,并利用其细胞内转导信号功能触发U87胶质母细胞瘤癌细胞的自噬介导的细胞死亡。ARP101抑制proMMP-2活化并触发MT1-MMP寡聚物的形成,这需要转运到质膜上。ARP101进一步诱导了细胞自噬,这反映为酸性液泡细胞器,LC3泪点和自噬相关蛋白ATG9转录的形成增加。当siRNA介导的MT1-MMP基因沉默时,所有这些都被显着逆转。因此,ARP101可以同时抑制MT1-MMP的细胞外催化功能,并利用其细胞内转导信号功能触发U87胶质母细胞瘤癌细胞的自噬介导的细胞死亡。ARP101抑制proMMP-2活化并触发MT1-MMP寡聚物的形成,这需要转运到质膜上。ARP101进一步诱导了细胞自噬,这反映为酸性液泡细胞器,LC3泪点和自噬相关蛋白ATG9转录的形成增加。当siRNA介导的MT1-MMP基因沉默时,所有这些都被显着逆转。因此,ARP101可以同时抑制MT1-MMP的细胞外催化功能,并利用其细胞内转导信号功能触发U87胶质母细胞瘤癌细胞的自噬介导的细胞死亡。当siRNA介导的MT1-MMP基因沉默时,所有这些都被显着逆转。因此,ARP101可以同时抑制MT1-MMP的细胞外催化功能,并利用其细胞内转导信号功能触发U87胶质母细胞瘤癌细胞的自噬介导的细胞死亡。当siRNA介导的MT1-MMP基因沉默时,所有这些都被显着逆转。因此,ARP101可以同时抑制MT1-MMP的细胞外催化功能,并利用其细胞内转导信号功能触发U87胶质母细胞瘤癌细胞的自噬介导的细胞死亡。
更新日期:2018-10-30
中文翻译:

ARP101靶向膜1型基质金属蛋白酶的双重功能:对U87胶质母细胞瘤细胞侵袭和自噬信号的影响。
膜1型基质金属蛋白酶(MT1-MMP)在肿瘤发生中同时具有细胞外蛋白水解和细胞内信号转导功能。MT1-MMP表达和/或功能失衡会触发转移性,侵袭性和治疗耐药性表型。MT1-MMP参与细胞外基质(ECM)的蛋白水解,潜在MMP的激活以及人类肝癌和胶质母细胞瘤细胞的自噬信号传导。最近的研究推断,在肿瘤发生过程中自噬指数低,其中自噬能力在肿瘤进展过程中降低。在这里,我们建立ARP101作为双功能小分子抑制剂,针对IV级胶质母细胞瘤细胞模型中的MT1-MMP ECM水解和自噬信号转导功能。ARP101抑制伴刀豆球蛋白A介导的proMMP-2活化为MMP-2,以及MT1-MMP自动蛋白水解处理。当过表达重组Wt MT1-MMP时,ARP101抑制proMMP-2激活并触发MT1-MMP寡聚体的形成,从而需要转运至质膜。ARP101进一步诱导了细胞自噬,这反映为酸性液泡细胞器,LC3泪点和自噬相关蛋白ATG9转录的形成增加。当siRNA介导的MT1-MMP基因沉默时,所有这些都被显着逆转。因此,ARP101可以同时抑制MT1-MMP的细胞外催化功能,并利用其细胞内转导信号功能触发U87胶质母细胞瘤癌细胞的自噬介导的细胞死亡。ARP101抑制proMMP-2活化并触发MT1-MMP寡聚物的形成,这需要转运到质膜上。ARP101进一步诱导了细胞自噬,这反映为酸性液泡细胞器,LC3泪点和自噬相关蛋白ATG9转录的形成增加。当siRNA介导的MT1-MMP基因沉默时,所有这些都被显着逆转。因此,ARP101可以同时抑制MT1-MMP的细胞外催化功能,并利用其细胞内转导信号功能触发U87胶质母细胞瘤癌细胞的自噬介导的细胞死亡。ARP101抑制proMMP-2活化并触发MT1-MMP寡聚物的形成,这需要转运到质膜上。ARP101进一步诱导了细胞自噬,这反映为酸性液泡细胞器,LC3泪点和自噬相关蛋白ATG9转录的形成增加。当siRNA介导的MT1-MMP基因沉默时,所有这些都被显着逆转。因此,ARP101可以同时抑制MT1-MMP的细胞外催化功能,并利用其细胞内转导信号功能触发U87胶质母细胞瘤癌细胞的自噬介导的细胞死亡。当siRNA介导的MT1-MMP基因沉默时,所有这些都被显着逆转。因此,ARP101可以同时抑制MT1-MMP的细胞外催化功能,并利用其细胞内转导信号功能触发U87胶质母细胞瘤癌细胞的自噬介导的细胞死亡。当siRNA介导的MT1-MMP基因沉默时,所有这些都被显着逆转。因此,ARP101可以同时抑制MT1-MMP的细胞外催化功能,并利用其细胞内转导信号功能触发U87胶质母细胞瘤癌细胞的自噬介导的细胞死亡。









































 京公网安备 11010802027423号
京公网安备 11010802027423号