当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Enantiospecific Borylation for Chiral α‐Amino Tertiary Boronic Esters
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-19 , DOI: 10.1002/anie.201809389 Qingqing Qi 1 , Xuena Yang 1 , Xiaoping Fu 1 , Shiqing Xu 1 , Ei‐ichi Negishi 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-19 , DOI: 10.1002/anie.201809389 Qingqing Qi 1 , Xuena Yang 1 , Xiaoping Fu 1 , Shiqing Xu 1 , Ei‐ichi Negishi 1
Affiliation
|
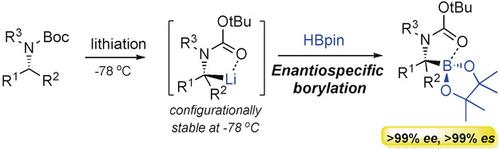
Herein we report a highly efficient and enantiospecific borylation method to synthesize a wide range of enantiopure (>99 % ee) α‐amino tertiary boronic esters. The configurationally stable α‐N‐Boc substituted tertiary organolithium species and pinacolborane (HBpin) underwent enantiospecific borylation at −78 °C with the formation of a new stereogenic C−B bond. This reaction has a broad scope, enabling the synthesis of various α‐amino tertiary boronic esters in excellent yields and, importantly, with universally excellent enantiospecificity (>99 % es) and complete retention of configuration.
中文翻译:

手性α-氨基叔硼酸酯的高度对映体硼化
在此,我们报道了一种高效且对映体特异性的硼酸酯化方法,可合成多种对映纯(> 99% ee)α-氨基叔硼酸酯。构型稳定的α-N-Boc取代的叔有机锂物质和频哪醇硼烷(HBpin)在-78°C下进行对映体特异性硼化反应,并形成新的立体异构C-B键。该反应范围广泛,能够以优异的产率合成各种α-氨基叔硼酸酯,重要的是,具有普遍优异的对映体特异性(> 99%es)并完全保留构型。
更新日期:2018-10-19
中文翻译:

手性α-氨基叔硼酸酯的高度对映体硼化
在此,我们报道了一种高效且对映体特异性的硼酸酯化方法,可合成多种对映纯(> 99% ee)α-氨基叔硼酸酯。构型稳定的α-N-Boc取代的叔有机锂物质和频哪醇硼烷(HBpin)在-78°C下进行对映体特异性硼化反应,并形成新的立体异构C-B键。该反应范围广泛,能够以优异的产率合成各种α-氨基叔硼酸酯,重要的是,具有普遍优异的对映体特异性(> 99%es)并完全保留构型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号