当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Behavior of the Anionic Surfactant [Bmim][AOT]-Stabilized Hydrophobic Ionic Liquid-Based Microemulsions and the Effect of n-Alcohols
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-10-17 , DOI: 10.1021/acs.iecr.8b03766 Rongrong Wang 1 , Zhenyu Feng 1 , Wei Jin 1 , Xirong Huang 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-10-17 , DOI: 10.1021/acs.iecr.8b03766 Rongrong Wang 1 , Zhenyu Feng 1 , Wei Jin 1 , Xirong Huang 1
Affiliation
|
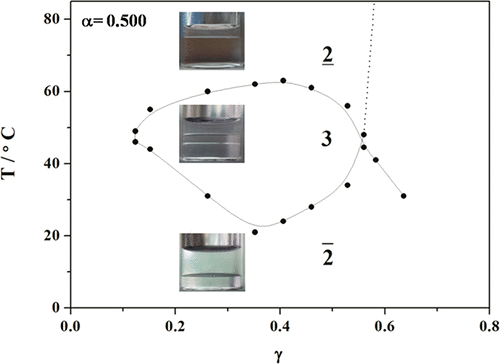
In this work, the fishlike phase diagram of the H2O/[Bmim][AOT]/[Bmim][PF6]/n-alcohol as a function of temperature (T) and the mass fraction of [Bmim][AOT] (with or without n-alcohol) in the total mixture (γ) has been observed for the first time at several mass ratios of [Bmim][PF6] to H2O (α) and with different n-alcohols. The larger area of the three-phase region occurs at α ≤ 0.500, and the resulting fish shapes are similar to each other. For a given α, a temperature scan (from lower to higher) at several γ values reveals that the present system forms an upper phase microemulsion first and then a lower phase microemulsion. The formation of hydrophobic ionic liquid-in-water (HIL/W) microemulsion at low temperature and water-in-hydrophobic ionic liquid (W/HIL) microemulsion at high temperature was confirmed by dynamic light scattering and small-angle X-ray scattering techniques. Here, the phase sequence that occurred during the temperature scan is opposite to that of a classic H2O/NaAOT/oil system. At the lower temperature, the H-bonding interaction is considered to be the main driving force for the aggregation; at the higher temperature, however, the main driving force may be the hydrophobic interaction. n-Alcohols with medium/long alkyl chains have a great influence on the fish-tail coordinates of the present systems. Compared with the ternary system without alcohol, the addition of n-alcohols (C4–C8) decreases the phase inversion temperature (T̃) and the surfactant efficiency. With the increase of the alkyl chain length of n-alcohols, however, the decrement in T̃ becomes smaller due to the increase of the interfacial rigidity. A comparison of these results with those obtained for the H2O/NaAOT/oil system indicates that there are some similarities and also some differences, depending on the relative density, polarity, or hydrophobicity among the HIL, oil, and n-alcohols. The above insight into the phase behavior of the present HIL-based system helps to formulate biocompatible HIL-based AOT-stabilized microemulsions which are important templates for the biosynthesis of conducting polymers.
中文翻译:

阴离子表面活性剂[Bmim] [AOT]-稳定的疏水性离子液体微乳液的相行为和正醇的影响
在这项工作中,H 2 O / [Bmim] [AOT] / [Bmim] [PF 6 ] / n-醇的鱼状相图随温度(T)和[Bmim] [AOT]的质量分数变化](带有或不带ñ -醇)在总混合物(γ)已观察到首次在[BMIM] [PF的几个质量比6 ]至H 2 O(α)和具有不同ñ-酒精。三相区域的较大面积出现在α≤0.500,并且所形成的鱼形彼此相似。对于给定的α,在几个γ值处的温度扫描(从较低到较高)表明,本系统首先形成上相微乳液,然后形成下相微乳液。通过动态光散射和小角X射线散射证实了疏水性离子水包水型微乳液在低温下的形成和疏水性离子水包水型微乳液在高温下的形成。技术。在此,温度扫描期间发生的相序与经典H 2的相序相反O / NaAOT /油系统。在较低温度下,氢键相互作用被认为是聚集的主要驱动力。然而,在较高温度下,主要驱动力可能是疏水相互作用。具有中等/长烷基链的n-醇对本系统的鱼尾坐标具有很大的影响。与不含醇的三元体系相比,添加正醇(C 4 -C 8)降低了相转化温度(T̃)和表面活性剂效率。但是,随着正构醇烷基链长度的增加,T 3的减少。由于界面刚度的增加而变小。将这些结果与从H 2 O / NaAOT /油体系中获得的结果进行比较表明,取决于HIL,油和正醇之间的相对密度,极性或疏水性,存在一些相似之处和一些差异。对本发明的基于HIL的系统的相行为的以上了解有助于配制生物相容的基于HIL的AOT稳定的微乳液,其是用于导电聚合物的生物合成的重要模板。
更新日期:2018-10-18
中文翻译:

阴离子表面活性剂[Bmim] [AOT]-稳定的疏水性离子液体微乳液的相行为和正醇的影响
在这项工作中,H 2 O / [Bmim] [AOT] / [Bmim] [PF 6 ] / n-醇的鱼状相图随温度(T)和[Bmim] [AOT]的质量分数变化](带有或不带ñ -醇)在总混合物(γ)已观察到首次在[BMIM] [PF的几个质量比6 ]至H 2 O(α)和具有不同ñ-酒精。三相区域的较大面积出现在α≤0.500,并且所形成的鱼形彼此相似。对于给定的α,在几个γ值处的温度扫描(从较低到较高)表明,本系统首先形成上相微乳液,然后形成下相微乳液。通过动态光散射和小角X射线散射证实了疏水性离子水包水型微乳液在低温下的形成和疏水性离子水包水型微乳液在高温下的形成。技术。在此,温度扫描期间发生的相序与经典H 2的相序相反O / NaAOT /油系统。在较低温度下,氢键相互作用被认为是聚集的主要驱动力。然而,在较高温度下,主要驱动力可能是疏水相互作用。具有中等/长烷基链的n-醇对本系统的鱼尾坐标具有很大的影响。与不含醇的三元体系相比,添加正醇(C 4 -C 8)降低了相转化温度(T̃)和表面活性剂效率。但是,随着正构醇烷基链长度的增加,T 3的减少。由于界面刚度的增加而变小。将这些结果与从H 2 O / NaAOT /油体系中获得的结果进行比较表明,取决于HIL,油和正醇之间的相对密度,极性或疏水性,存在一些相似之处和一些差异。对本发明的基于HIL的系统的相行为的以上了解有助于配制生物相容的基于HIL的AOT稳定的微乳液,其是用于导电聚合物的生物合成的重要模板。











































 京公网安备 11010802027423号
京公网安备 11010802027423号