Current Opinion in Chemical Engineering ( IF 8.0 ) Pub Date : 2018-10-05 , DOI: 10.1016/j.coche.2018.08.009 Paul S Kelly , Antonio Alarcon Miguez , Christina Alves , Niall Barron
|
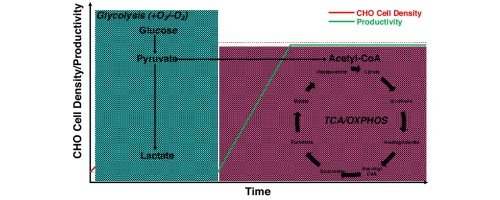
Meeting the metabolic demands of Chinese hamster ovary (CHO) cells has been an area of intense investigation over the last 3 decades as a means to improve these cell factories as producers of high quality recombinant therapeutic proteins. Metabolically, the cultivation of CHO cells is characterised by the rapid consumption of the primary carbon and energy sources, glucose and glutamine, with lactate and ammonia produced as by-products, respectively. In the context of bioprocess-relevant CHO cell phenotypes, glycolytic metabolism predominates during exponential cell growth culminating in lactate production while glucose channelling through the tri-carboxylic acid (TCA) cycle supports high-specific productivity. The genetic diversity inherent among CHO cell lineages (CHO-K1, CHO-S, and CHO-DG44), in addition to clonal isolates, makes media development a complex task which must often be performed on a clone by clone basis. However, designing tailored media formulations and sophisticated feeding regimens based on empirical observation has been one of the main driving forces behind the enhancements seen today in volumetric titres. To add to this complexity, CHO mitochondrial genetics have recently been shown to be heterogeneous resulting in an additional level of genetic pre-programming at the epicentre of cellular energy production. Cajoling CHO cells to utilise resources more efficiently through cell line development strategies, hypothermic adaptation or genetic engineering are areas of considerable interest within the biopharmaceutical community. Genetic re-programming of cellular metabolism through the manipulation of desirable metabolic pathways using microRNAs, siRNAs or gene overexpression have yielded some success. With the advent of sophisticated gene editing tools such as CRISPR-Cas9, a better understanding of CHO cell metabolism should drive knowledge-based multi-faceted cell line development pipelines combining both genetic engineering, selection of innately superior clones as well as tailored media formulations to improve the performance of this important therapeutic protein-producing cell line.










































 京公网安备 11010802027423号
京公网安备 11010802027423号