Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-10-06 , DOI: 10.1016/j.tetlet.2018.10.009 Tetsuro Murokawa , Masaru Enomoto , Takaaki Teranishi , Yusuke Ogura , Shigefumi Kuwahara
|
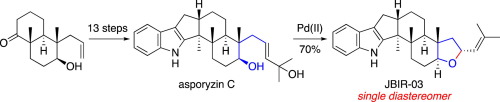
The first enantioselective total synthesis of JBIR-03 and asporyzin C, indole diterpenoids of fungal origin exhibiting a range of pharmacologically important biological activities, has been accomplished from a known bicyclic keto alcohol in 13 and 14 steps, respectively. A hydroxy-directed cyclopropanation and Pd(II)-mediated indole ring formation were exploited as the key steps to obtain a pivotal pentacylic intermediate, which was converted into asporyzin C via chain elongation using cross-metathesis and then into JBIR-03 by Pd(II)-catalyzed tetrahydrofuran ring formation in an exclusively diastereoselective manner.
中文翻译:

JBIR-03和Asporyzin C的全合成
JBIR-03和Asporyzin C(一种真菌来源的吲哚二萜类化合物,具有一系列药理上重要的生物活性)的首次对映选择性全合成已分别从已知的双环酮醇中分13步和14步完成。以羟基为导向的环丙烷化和Pd(II)介导的吲哚环的形成为关键步骤,以获取关键的五酰基中间体,该中间体通过交叉复分解通过链伸长转化为Asporyzin C,然后由Pd(J)转化为JBIR-03。 II)以非对映选择性的方式催化四氢呋喃环的形成。









































 京公网安备 11010802027423号
京公网安备 11010802027423号