当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Towards a continuous formic acid synthesis: a two-step carbon dioxide hydrogenation in flow†
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-10-04 00:00:00 , DOI: 10.1039/c8re00142a Helena Reymond 1, 2, 3, 4 , Juan José Corral-Pérez 5, 6, 7, 8 , Atsushi Urakawa 5, 6, 7, 8 , Philipp Rudolf von Rohr 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-10-04 00:00:00 , DOI: 10.1039/c8re00142a Helena Reymond 1, 2, 3, 4 , Juan José Corral-Pérez 5, 6, 7, 8 , Atsushi Urakawa 5, 6, 7, 8 , Philipp Rudolf von Rohr 1, 2, 3, 4
Affiliation
|
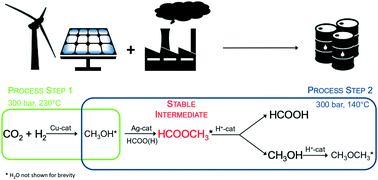
The need for long term, large-scale storage solutions to match surplus renewable energy with demand drives technological innovation towards a low-carbon economy. As a high hydrogen density energy carrier, formic acid streamlines functional storage of unscheduled intermittent power supply. However, the unfavourable thermodynamics of its direct synthesis from CO2 and H2 call for alternative processes to achieve substantial space time yields. This preliminary study investigates the feasibility of continuously producing formic acid in a two-step process by exploiting methyl formate as a thermodynamically stable intermediate. In order to prove the concept, the qualitative efficiency of several three-reactor configurations is evaluated and discussed with respect to the efficiency of a single reactor methanol synthesis over a commercial Cu catalyst. Although concrete solutions are not available yet and identification of formic acid remains arduous, the proposed reactive pathway exceeds the thermodynamic limits of the direct synthesis path over heterogeneous catalysts, and opens up avenues for advances in clean energy production.
中文翻译:

迈向连续的甲酸合成:流动中的两步式二氧化碳加氢†
需要长期,大规模的存储解决方案,以使过剩的可再生能源与需求相匹配,从而推动技术创新朝着低碳经济发展。作为高氢密度能量载体,甲酸简化了计划外间歇电源的功能存储。然而,由CO 2和H 2直接合成的不利的热力学要求采用替代方法以实现可观的时空产量。这项初步研究通过利用甲酸甲酯作为热力学稳定的中间体,研究了在两步法中连续生产甲酸的可行性。为了证明这一概念,就在商业铜催化剂上单反应器甲醇合成的效率进行了评估和讨论了几种三反应器配置的定性效率。尽管还没有具体的解决方案,而且甲酸的鉴定仍然很艰巨,但拟议的反应途径超过了异质催化剂上直接合成途径的热力学极限,并为清洁能源生产的发展开辟了道路。
更新日期:2018-10-04
中文翻译:

迈向连续的甲酸合成:流动中的两步式二氧化碳加氢†
需要长期,大规模的存储解决方案,以使过剩的可再生能源与需求相匹配,从而推动技术创新朝着低碳经济发展。作为高氢密度能量载体,甲酸简化了计划外间歇电源的功能存储。然而,由CO 2和H 2直接合成的不利的热力学要求采用替代方法以实现可观的时空产量。这项初步研究通过利用甲酸甲酯作为热力学稳定的中间体,研究了在两步法中连续生产甲酸的可行性。为了证明这一概念,就在商业铜催化剂上单反应器甲醇合成的效率进行了评估和讨论了几种三反应器配置的定性效率。尽管还没有具体的解决方案,而且甲酸的鉴定仍然很艰巨,但拟议的反应途径超过了异质催化剂上直接合成途径的热力学极限,并为清洁能源生产的发展开辟了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号