当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing the Delicate Balance Between Pauli Repulsion and London Dispersion with Triphenylmethyl Derivatives
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-10-05 , DOI: 10.1021/jacs.8b09145 Sören Rösel 1 , Jonathan Becker 2 , Wesley D. Allen , Peter R. Schreiner 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-10-05 , DOI: 10.1021/jacs.8b09145 Sören Rösel 1 , Jonathan Becker 2 , Wesley D. Allen , Peter R. Schreiner 1
Affiliation
|
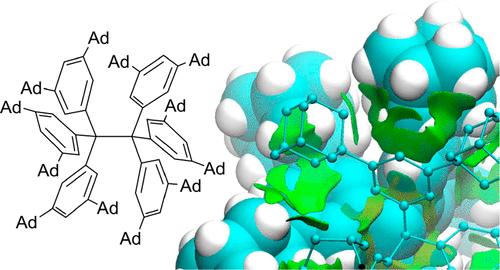
The long-known, ubiquitously present, and always attractive London dispersion (LD) interaction was probed with hexaphenylethane (HPE) derivatives. A series of all- meta hydrocarbyl [Me, iPr, tBu, Cy, Ph, 1-adamantyl (Ad)]-substituted triphenylmethyl (TPM) derivatives [TPM-H, TPM-OH, (TPM-O)2, TPM•] was synthesized en route, and several derivatives were characterized by single-crystal X-ray diffraction (SC-XRD). Multiple dimeric head-to-head SC-XRD structures feature an excellent geometric fit between the meta-substituents; this is particularly true for the sterically most demanding tBu and Ad substituents. NMR spectra of the iPr-, tBu-, and Cy-derived trityl radicals were obtained and reveal, together with EPR and UV-Vis spectroscopic data, that the effects of all- meta alkyl substitution on the electronic properties of the trityl scaffold are marginal. Therefore, we concluded that the most important factor for HPE stability arises from LD interactions. Beyond all- meta tBu-HPE we also identified the hitherto unreported all- meta Ad-HPE. An intricate mathematical analysis of the temperature-dependent dissociation constants allowed us to extract Δ Gd298(exptl) = 0.3(5) kcal mol-1 from NMR experiments for all- meta tBu-HPE, in good agreement with previous experimental values and B3LYP-D3(BJ)/def2-TZVPP(C-PCM) computations. These computations show a stabilizing trend with substituent size in line with all- meta Ad-HPE (Δ Gd298(exptl) = 2.1(6) kcal mol-1) being more stable than its tBu congener. That is, large, rigid, and symmetric hydrocarbon moieties act as excellent dispersion energy donors. Provided a good geometric fit, they are able to stabilize labile molecules such as HPE via strong intramolecular LD interactions, even in solution.
中文翻译:

用三苯甲基衍生物探索泡利斥力和伦敦色散之间的微妙平衡
用六苯基乙烷 (HPE) 衍生物探测了众所周知的、无处不在且始终具有吸引力的伦敦分散 (LD) 相互作用。一系列全间位烃基 [Me, iPr, tBu, Cy, Ph, 1-金刚烷基 (Ad)]-取代的三苯甲基 (TPM) 衍生物 [TPM-H, TPM-OH, (TPM-O)2, TPM• ] 在途中合成,并通过单晶 X 射线衍射 (SC-XRD) 表征了几种衍生物。多个二聚体头对头 SC-XRD 结构在元取代基之间具有出色的几何拟合;对于空间上要求最高的 tBu 和 Ad 取代基尤其如此。获得并揭示了 iPr-、tBu-和 Cy 衍生的三苯甲基自由基的 NMR 光谱,以及 EPR 和 UV-Vis 光谱数据,所有间位烷基取代对三苯甲基支架的电子特性的影响是微不足道的。因此,我们得出结论,HPE 稳定性的最重要因素来自 LD 相互作用。除了全元 tBu-HPE,我们还确定了迄今为止未报告的全元 Ad-HPE。温度相关解离常数的复杂数学分析使我们能够从全元 tBu-HPE 的 NMR 实验中提取 Δ Gd298(exptl) = 0.3(5) kcal mol-1,与之前的实验值和 B3LYP- D3(BJ)/def2-TZVPP(C-PCM) 计算。这些计算显示出稳定趋势,取代基大小与全元 Ad-HPE 一致(Δ Gd298(exptl) = 2.1(6) kcal mol-1)比其 tBu 同系物更稳定。也就是说,大的,刚性的,和对称烃部分作为优良的分散能量供体。提供良好的几何拟合,它们能够通过强大的分子内 LD 相互作用稳定不稳定的分子,例如 HPE,即使在溶液中也是如此。
更新日期:2018-10-05
中文翻译:

用三苯甲基衍生物探索泡利斥力和伦敦色散之间的微妙平衡
用六苯基乙烷 (HPE) 衍生物探测了众所周知的、无处不在且始终具有吸引力的伦敦分散 (LD) 相互作用。一系列全间位烃基 [Me, iPr, tBu, Cy, Ph, 1-金刚烷基 (Ad)]-取代的三苯甲基 (TPM) 衍生物 [TPM-H, TPM-OH, (TPM-O)2, TPM• ] 在途中合成,并通过单晶 X 射线衍射 (SC-XRD) 表征了几种衍生物。多个二聚体头对头 SC-XRD 结构在元取代基之间具有出色的几何拟合;对于空间上要求最高的 tBu 和 Ad 取代基尤其如此。获得并揭示了 iPr-、tBu-和 Cy 衍生的三苯甲基自由基的 NMR 光谱,以及 EPR 和 UV-Vis 光谱数据,所有间位烷基取代对三苯甲基支架的电子特性的影响是微不足道的。因此,我们得出结论,HPE 稳定性的最重要因素来自 LD 相互作用。除了全元 tBu-HPE,我们还确定了迄今为止未报告的全元 Ad-HPE。温度相关解离常数的复杂数学分析使我们能够从全元 tBu-HPE 的 NMR 实验中提取 Δ Gd298(exptl) = 0.3(5) kcal mol-1,与之前的实验值和 B3LYP- D3(BJ)/def2-TZVPP(C-PCM) 计算。这些计算显示出稳定趋势,取代基大小与全元 Ad-HPE 一致(Δ Gd298(exptl) = 2.1(6) kcal mol-1)比其 tBu 同系物更稳定。也就是说,大的,刚性的,和对称烃部分作为优良的分散能量供体。提供良好的几何拟合,它们能够通过强大的分子内 LD 相互作用稳定不稳定的分子,例如 HPE,即使在溶液中也是如此。



























 京公网安备 11010802027423号
京公网安备 11010802027423号