当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formaldehyde–isobutene Prins condensation over MFI-type zeolites†
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-10-04 00:00:00 , DOI: 10.1039/c8cy01667d Efterpi S. Vasiliadou 1, 2, 3, 4, 5 , Sha Li 1, 2, 3, 4, 5 , Stavros Caratzoulas 1, 2, 3, 4, 5 , Raul F. Lobo 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2018-10-04 00:00:00 , DOI: 10.1039/c8cy01667d Efterpi S. Vasiliadou 1, 2, 3, 4, 5 , Sha Li 1, 2, 3, 4, 5 , Stavros Caratzoulas 1, 2, 3, 4, 5 , Raul F. Lobo 1, 2, 3, 4, 5
Affiliation
|
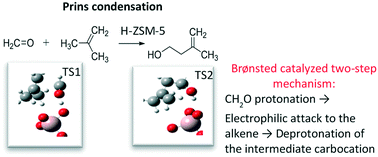
The liquid phase Prins condensation of formaldehyde with butenes on H-ZSM-5 (MFI) zeolite catalysts was investigated showing that reaction rates follow the order isobutene > 1-butene > cis-2-butene. The catalytic rates for medium-pore zeolite H-ZSM-5 were not only a larger than for H-beta, but also selective for 3-methyl-3-buten-1-ol during formaldehyde condensation with isobutene. Isoprene forms, under the optimal reaction conditions, either in a second dehydration step of the unsaturated alcohol, or in a single step over H-ZSM-5 with optimal Si/Al ratio (Si/Al = 40). Mechanistic studies revealed that sequential Prins cyclization and hetero-Diels–Alder reactions of the desired products, forming six carbon species, occur only at slow rates over H-ZSM-5. Using DFT methods it was determined that the reaction follows a two-step mechanism: protonation of formaldehyde and electrophilic attack to the alkene, and deprotonation of the resulting intermediate carbocation. For isobutene and 1-butene, the electrophilic addition is coordinated with the protonation of the formyl group, but for cis-2-butene, it is coordinated with the proton back-donation step. The rate-limiting step switches from electrophilic addition for isobutene to proton elimination for the other two C4 isomers.
中文翻译:

甲醛-异丁烯树脂在MFI型沸石上的缩合†
研究了H-ZSM-5(MFI)沸石催化剂上甲醛与丁烯的液相Prins缩合反应,显示反应速率遵循异丁烯> 1-丁烯>顺式的顺序-2-丁烯。中孔沸石H-ZSM-5的催化速率不仅大于H-β,而且在甲醛与异丁烯缩合过程中对3-甲基-3-丁烯-1-醇具有选择性。在最佳反应条件下,异戊二烯要么在不饱和醇的第二个脱水步骤中形成,要么在具有最佳Si / Al比(Si / Al = 40)的H-ZSM-5上一步形成。机理研究表明,所需产物的顺序Prins环化反应和杂Diels-Alder反应(形成六个碳原子)仅在H-ZSM-5上以较慢的速率发生。使用DFT方法,确定该反应遵循两步机理:甲醛的质子化和对烯烃的亲电攻击,以及所得中间体碳正离子的去质子化。对于异丁烯和1-丁烯,顺式-2-丁烯,它与质子返还步骤协调。限速步骤从对异丁烯的亲电加成转换为对其他两个C4异构体的质子消除。
更新日期:2018-10-04
中文翻译:

甲醛-异丁烯树脂在MFI型沸石上的缩合†
研究了H-ZSM-5(MFI)沸石催化剂上甲醛与丁烯的液相Prins缩合反应,显示反应速率遵循异丁烯> 1-丁烯>顺式的顺序-2-丁烯。中孔沸石H-ZSM-5的催化速率不仅大于H-β,而且在甲醛与异丁烯缩合过程中对3-甲基-3-丁烯-1-醇具有选择性。在最佳反应条件下,异戊二烯要么在不饱和醇的第二个脱水步骤中形成,要么在具有最佳Si / Al比(Si / Al = 40)的H-ZSM-5上一步形成。机理研究表明,所需产物的顺序Prins环化反应和杂Diels-Alder反应(形成六个碳原子)仅在H-ZSM-5上以较慢的速率发生。使用DFT方法,确定该反应遵循两步机理:甲醛的质子化和对烯烃的亲电攻击,以及所得中间体碳正离子的去质子化。对于异丁烯和1-丁烯,顺式-2-丁烯,它与质子返还步骤协调。限速步骤从对异丁烯的亲电加成转换为对其他两个C4异构体的质子消除。











































 京公网安备 11010802027423号
京公网安备 11010802027423号