Theranostics ( IF 12.4 ) Pub Date : 2018-10-05 , DOI: 10.7150/thno.28344 Huan Liang , Zhanwei Zhou , Renjie Luo , Mangmang Sang , Bowen Liu , Minjie Sun , Wei Qu , Feng Feng , Wenyuan Liu
|
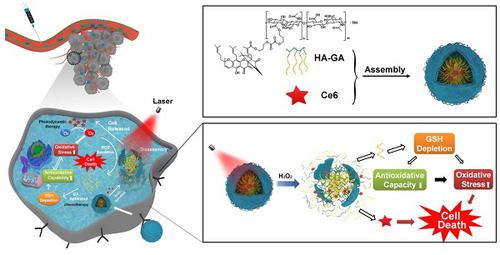
Photodynamic therapy relies on photosensitizers to generate cytotoxic reactive oxygen species (ROS) resulting in the apoptois of tumor cells. However, there is an antioxidant system that impedes the elevation of oxidation levels in tumor cells. Thus, photodynamic therapy may exhibit insufficient curative effects due to ungenerous reactive oxygen species levels. Herein, we describe tumor-specific activated photodynamic therapy using an oxidation-regulating strategy.
Methods: We first synthesised a reactive oxygen species-sensitive amphipathic prodrug of gambogic acid-grafted hyaluronic acid (HA-GA). The hydrophobic photosensitizer chlorin e6 (Ce6) was then loaded into HA-GA by hydrophobic interactions between GA and Ce6, forming amphipathic nanomicelles (HA-GA@Ce6). The ROS-responsive behavior, cytotoxicity, cell uptake, tumor cell killing, in vivo biodistribution and in vivo anti-tumor efficacy of HA-GA@Ce6 were investigated. The in vitro and in vivo experiments were performed on 4T1 murine breast cancer cells and 4T1 tumor model.
Results: We validated that the micelles of HA-GA@Ce6 showed stronger cell uptake in 4T1 tumor cells and lower cytotoxicity in normal cells compared with free Ce6 and GA, which exhibited the benefits of nanomicelles on enhancing the tumor cell acumulation and reducing the side effects on normal cells synchronously. Additionally, the cytotoxic free radicals of photodynamic therapy were generated after irradiation and the high oxidation levels activated the ROS-sensitive GA prodrug efficiently, which killed the tumor cells and depleted intracellular glutathione (GSH), thereby impairing antioxidant levels and enhancing photodynamic therapy.
Conclusion: With the successfully eradicated tumor growth in vivo. Our work represents a new photodynamic therapy concept, achieving superior anti-tumor efficacy by reducing intracellular antioxidant levels.
Keywords: chlorin e6, gambogic acid, photodynamic therapy, ROS-sensitive, GSH depletion
中文翻译:

具有氧化调节策略的肿瘤特异性活化光动力疗法,可增强抗肿瘤功效
光动力疗法依靠光敏剂产生细胞毒性的活性氧(ROS),导致肿瘤细胞的凋亡。但是,有一种抗氧化剂系统会阻止肿瘤细胞中氧化水平的升高。因此,光动力疗法可能由于未反应的活性氧种类水平而显示出不足的疗效。在这里,我们描述了使用氧化调节策略的肿瘤特异性活化光动力疗法。
方法:我们首先合成了藤黄酸接枝的透明质酸(HA-GA)对活性氧物种敏感的两亲性前药。然后通过GA和Ce6之间的疏水相互作用将疏水性光敏剂二氢卟酚e6(Ce6)加载到HA-GA中,形成两亲纳米胶束(HA-GA @ Ce6)。研究了HA-GA @ Ce6的ROS响应行为,细胞毒性,细胞摄取,肿瘤细胞杀伤,体内生物分布和体内抗肿瘤功效。在体外和体内实验是在4T1鼠乳腺癌细胞和4T1肿瘤模型中进行。
结果:我们证实,与游离的Ce6和GA相比,HA-GA @ Ce6的胶束在4T1肿瘤细胞中显示出更强的细胞摄取,在正常细胞中具有较低的细胞毒性,这表明纳米胶束在增强肿瘤细胞的聚集和减少侧面方面具有优势同步影响正常细胞。此外,光动力疗法的细胞毒性自由基在照射后产生,高氧化水平有效激活了ROS敏感的GA前药,杀死了肿瘤细胞并耗尽了细胞内的谷胱甘肽(GSH),从而削弱了抗氧化剂的水平并增强了光动力疗法。
结论:随着体内肿瘤的成功根除。我们的工作代表了一种新的光动力疗法概念,可通过降低细胞内抗氧化剂水平来实现卓越的抗肿瘤功效。
关键词:二氢卟酚e6,藤黄酸,光动力疗法,ROS敏感,谷胱甘肽耗竭



























 京公网安备 11010802027423号
京公网安备 11010802027423号