当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective Nitrative Cyclization of 1,6-Enynes with t-BuONO under Metal-Free Conditions
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-10-04 00:00:00 , DOI: 10.1021/acssuschemeng.8b03752 Wen-Ting Wei 1 , Wei-Wei Ying 1 , Wen-Hui Bao 1 , Le-Han Gao 1 , Xu-Dong Xu 1 , Yi-Ning Wang 1 , Xiao-Xiao Meng 1 , Gan-Ping Chen 1 , Qiang Li 2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-10-04 00:00:00 , DOI: 10.1021/acssuschemeng.8b03752 Wen-Ting Wei 1 , Wei-Wei Ying 1 , Wen-Hui Bao 1 , Le-Han Gao 1 , Xu-Dong Xu 1 , Yi-Ning Wang 1 , Xiao-Xiao Meng 1 , Gan-Ping Chen 1 , Qiang Li 2
Affiliation
|
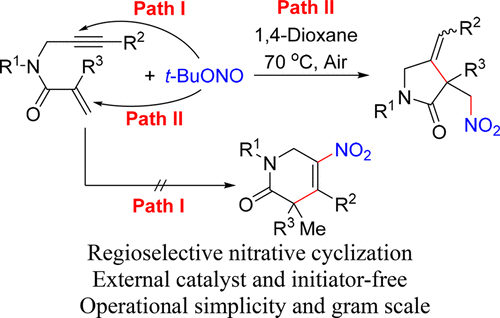
A new pattern for nitrative cyclization of 1,6-enynes with t-BuONO has been reported for the synthesis of various 2-pyrrolidinone derivatives in 45–88% yields. This novel method is operationally simple and proceeds under very mild conditions without using any additives. The reaction pathway involves nitro radical addition toward an alkenyl group/5-exo-cyclization/H-abstraction sequence, allowing a highly regioselective and practical protocol toward the formation of new C–N and C–C bonds.
中文翻译:

在无金属条件下用t -BuONO进行1,6-烯炔的区域选择性硝化环化
已经报道了一种用叔-BuONO对1,6-烯炔进行硝化环化的新模式,用于合成各种2-吡咯烷酮衍生物,产率为45-88%。该新颖方法操作简单,并且在非常温和的条件下进行而无需使用任何添加剂。该反应途径涉及向烯基/ 5-外-环化/ H-抽象序列添加硝基自由基,从而为形成新的C-N和CC键提供了高度区域选择性和实用的方法。
更新日期:2018-10-04
中文翻译:

在无金属条件下用t -BuONO进行1,6-烯炔的区域选择性硝化环化
已经报道了一种用叔-BuONO对1,6-烯炔进行硝化环化的新模式,用于合成各种2-吡咯烷酮衍生物,产率为45-88%。该新颖方法操作简单,并且在非常温和的条件下进行而无需使用任何添加剂。该反应途径涉及向烯基/ 5-外-环化/ H-抽象序列添加硝基自由基,从而为形成新的C-N和CC键提供了高度区域选择性和实用的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号