当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Prediction of Mefenamic Acid Solubility and Molecular Interaction Energies in Different Classes of Organic Solvents and Water
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-10-17 , DOI: 10.1021/acs.iecr.8b02722 Siti K. Abdul Mudalip 1 , Mohd R. Abu Bakar 2 , P. Jamal 3 , F. Adam 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-10-17 , DOI: 10.1021/acs.iecr.8b02722 Siti K. Abdul Mudalip 1 , Mohd R. Abu Bakar 2 , P. Jamal 3 , F. Adam 1
Affiliation
|
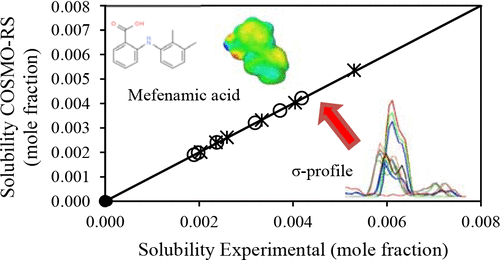
Determination of solubility data either through experimental- or model-based approaches becomes a necessity in the crystallization of pharmaceutical compounds. The current work predicts the mefenamic acid solubility and molecular interaction energy, namely electrostatic (H-MF), hydrogen bonding (H-HB), and van der Waals (H-vdW), in different solvents at temperatures from 298 to 323 K using the conductor-like screening model for real solvents (COSMO-RS). The solvents used were N,N-dimethylacetamide, N,N-dimethylformamide, acetone, ethyl acetate, ethanol, iso-propyl alcohol, n-hexane, n-heptane, cyclohexane, and water. The Gibbs free energy of fusion required in COSMO-RS computation was determined using differential scanning calorimetry and reference solubility method. The accuracy of methods employed in prediction of solubility were evaluated using mean squared quadratic error (MSE). The mefenamic acid solubility predicted using COSMO-RS with reference solubility method showed a small MSE value of less than 2%. The predicted solubility also follows the same trend as the experimental values and increases with temperature. The predicted H-HB energy and Gibbs free energy changes of mefenamic acid dissolution in the solvents studied highly influence the solubility data. Therefore, COSMO-RS with reference solubility method is a promising approach to predict the solubility and intermolecular interaction energy of mefenamic acid in different solvents.
中文翻译:

不同类别有机溶剂和水中甲芬那酸的溶解度和分子相互作用能的预测
通过实验或基于模型的方法确定溶解度数据成为药物化合物结晶的必要条件。当前的工作预测了在298至323 K的温度下,在不同溶剂中的甲芬那酸溶解度和分子相互作用能,即静电(H-MF),氢键(H-HB)和范德华力(H-vdW)。真实溶剂的类似导体的筛选模型(COSMO-RS)。使用的溶剂是N,N-二甲基乙酰胺,N,N-二甲基甲酰胺,丙酮,乙酸乙酯,乙醇,异丙基醇,Ñ己烷,Ñ庚烷,环己烷和水。使用差示扫描量热法和参考溶解度法确定了COSMO-RS计算中所需的吉布斯聚变自由能。使用均方二次误差(MSE)评估了用于预测溶解度的方法的准确性。使用COSMO-RS和参考溶解度方法预测的甲芬那酸溶解度显示出小于2%的小MSE值。预测的溶解度也遵循与实验值相同的趋势,并随温度的升高而增加。预测的甲芬那酸在溶剂中溶解的H-HB能量和吉布斯自由能变化对溶解度数据的影响很大。所以,
更新日期:2018-10-18
中文翻译:

不同类别有机溶剂和水中甲芬那酸的溶解度和分子相互作用能的预测
通过实验或基于模型的方法确定溶解度数据成为药物化合物结晶的必要条件。当前的工作预测了在298至323 K的温度下,在不同溶剂中的甲芬那酸溶解度和分子相互作用能,即静电(H-MF),氢键(H-HB)和范德华力(H-vdW)。真实溶剂的类似导体的筛选模型(COSMO-RS)。使用的溶剂是N,N-二甲基乙酰胺,N,N-二甲基甲酰胺,丙酮,乙酸乙酯,乙醇,异丙基醇,Ñ己烷,Ñ庚烷,环己烷和水。使用差示扫描量热法和参考溶解度法确定了COSMO-RS计算中所需的吉布斯聚变自由能。使用均方二次误差(MSE)评估了用于预测溶解度的方法的准确性。使用COSMO-RS和参考溶解度方法预测的甲芬那酸溶解度显示出小于2%的小MSE值。预测的溶解度也遵循与实验值相同的趋势,并随温度的升高而增加。预测的甲芬那酸在溶剂中溶解的H-HB能量和吉布斯自由能变化对溶解度数据的影响很大。所以,











































 京公网安备 11010802027423号
京公网安备 11010802027423号