当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Study of the N-Formylation of Amines with Carbon Dioxide and Hydrosilanes
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-04 00:00:00 , DOI: 10.1021/acscatal.8b03274 Martin Hulla 1 , Gabor Laurenczy 1 , Paul J. Dyson 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-04 00:00:00 , DOI: 10.1021/acscatal.8b03274 Martin Hulla 1 , Gabor Laurenczy 1 , Paul J. Dyson 1
Affiliation
|
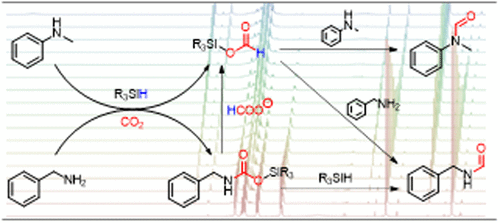
N-formylation of amines with CO2 and hydrosilane reducing agents proceeds via fast and complex chemical equilibria, which hinder easy analysis of the reaction pathways. In situ reaction monitoring and kinetic studies reveal that three proposed pathways, via direct- and amine-assisted formoxysilane formation (pathways 1 and 2, respectively) and via a silylcarbamate intermediate (pathway 3), are possible depending on the reaction conditions and the substrates. While pathway 1 is favored for non-nucleophilic amines in the absence of a catalyst, a base catalyst results in noninnocent behavior of the amine in the CO2 reduction step toward the formoxysilane intermediate. The reaction pathway is altered by strongly nucleophilic amines, which form stable adducts with CO2. Silylcarbamate intermediates form, which can be directly reduced to the N-formylated products by excess hydrosilane. Nevertheless, without excess hydrosilane, the silylcarbamate is an additional intermediate en route to formoxysilanes along pathway 2. Exchange NMR spectroscopy (EXSY) revealed extensive substituent exchange around the hydrosilane silicon center, which confirms its activation during the reaction and supports the proposed reaction mechanisms. Numerous side reactions were also identified, which help to establish the reaction equilibria in the N-formylation reactions.
中文翻译:

胺与二氧化碳和氢硅烷的N-甲酰化反应机理的研究
胺与CO 2和氢硅烷还原剂的N-甲酰化反应通过快速而复杂的化学平衡进行,这阻碍了对反应路径的轻松分析。原位反应监测和动力学研究表明,取决于反应条件和底物,可能通过直接和胺辅助的甲氧基硅烷形成(分别为途径1和2)以及通过甲硅烷基氨基甲酸酯中间体(途径3)提出三种提议的途径。 。尽管在不存在催化剂的情况下,路径1对于非亲核胺是有利的,但碱催化剂会导致胺在CO 2还原步骤中朝着甲氧基硅烷中间体的无毒行为。反应路径被强亲核胺所改变,该亲核胺与CO 2形成稳定的加合物。形成氨基甲酸酯甲硅烷基中间体,可以通过过量的氢硅烷将其直接还原为N-甲酰化产物。然而,在没有过量的氢硅烷的情况下,甲硅烷基氨基甲酸酯是沿着途径2形成甲氧基硅烷的另一种中间产物。交换NMR光谱(EXSY)揭示了氢硅烷中心附近的大量取代基交换,这证实了其在反应过程中的活化并支持了所提出的反应机理。还确定了许多副反应,这有助于在N-甲酰化反应中建立反应平衡。
更新日期:2018-10-04
中文翻译:

胺与二氧化碳和氢硅烷的N-甲酰化反应机理的研究
胺与CO 2和氢硅烷还原剂的N-甲酰化反应通过快速而复杂的化学平衡进行,这阻碍了对反应路径的轻松分析。原位反应监测和动力学研究表明,取决于反应条件和底物,可能通过直接和胺辅助的甲氧基硅烷形成(分别为途径1和2)以及通过甲硅烷基氨基甲酸酯中间体(途径3)提出三种提议的途径。 。尽管在不存在催化剂的情况下,路径1对于非亲核胺是有利的,但碱催化剂会导致胺在CO 2还原步骤中朝着甲氧基硅烷中间体的无毒行为。反应路径被强亲核胺所改变,该亲核胺与CO 2形成稳定的加合物。形成氨基甲酸酯甲硅烷基中间体,可以通过过量的氢硅烷将其直接还原为N-甲酰化产物。然而,在没有过量的氢硅烷的情况下,甲硅烷基氨基甲酸酯是沿着途径2形成甲氧基硅烷的另一种中间产物。交换NMR光谱(EXSY)揭示了氢硅烷中心附近的大量取代基交换,这证实了其在反应过程中的活化并支持了所提出的反应机理。还确定了许多副反应,这有助于在N-甲酰化反应中建立反应平衡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号