当前位置:
X-MOL 学术
›
Macromol. Rapid Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Multifunctional Homopolymers through Using Thiazolidine Chemistry and Post‐Polymerization Modification
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2018-10-03 , DOI: 10.1002/marc.201800590 Tomohiro Kubo 1 , Jeremy L. Swartz 1 , Georg M. Scheutz 1 , Brent S. Sumerlin 1
Macromolecular Rapid Communications ( IF 4.2 ) Pub Date : 2018-10-03 , DOI: 10.1002/marc.201800590 Tomohiro Kubo 1 , Jeremy L. Swartz 1 , Georg M. Scheutz 1 , Brent S. Sumerlin 1
Affiliation
|
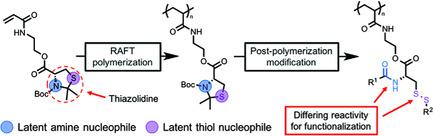
Multifunctional homopolymers, defined here as polymers that contain multiple reactive functional groups per repeat unit, are versatile scaffolds for preparing complex macromolecules via post‐polymerization modification. However, there are limited methods for preparing multifunctional homopolymers that contain more than one nucleophilic site per repeat unit. Herein, a strategy to synthesize a multifunctional homopolymer using thiazolidine chemistry is demonstrated. Controlled radical polymerization of a thiazolidine‐containing acrylamido monomer allows for the synthesis of a polymer with pendent latent nucleophiles. Ring‐opening of the thiazolidine affords a homopolymer with two side‐chain reactive sites, an amine and a thiol. One‐pot functionalization via disulfide formation and acyl substitution is performed to introduce two distinct groups in each repeat unit.
中文翻译:

噻唑烷化学和聚合后改性合成多功能均聚物
多功能均聚物,这里定义为每个重复单元包含多个反应性官能团的聚合物,是一种多功能的支架,用于通过聚合后修饰制备复杂的大分子。然而,用于制备每个重复单元包含一个以上亲核位点的多功能均聚物的方法有限。在本文中,证明了使用噻唑烷化学合成多功能均聚物的策略。含噻唑烷的丙烯酰胺基单体的受控自由基聚合可合成具有潜在潜在亲核试剂的聚合物。噻唑烷的开环得到具有两个侧链反应位点,胺和硫醇的均聚物。
更新日期:2018-10-03
中文翻译:

噻唑烷化学和聚合后改性合成多功能均聚物
多功能均聚物,这里定义为每个重复单元包含多个反应性官能团的聚合物,是一种多功能的支架,用于通过聚合后修饰制备复杂的大分子。然而,用于制备每个重复单元包含一个以上亲核位点的多功能均聚物的方法有限。在本文中,证明了使用噻唑烷化学合成多功能均聚物的策略。含噻唑烷的丙烯酰胺基单体的受控自由基聚合可合成具有潜在潜在亲核试剂的聚合物。噻唑烷的开环得到具有两个侧链反应位点,胺和硫醇的均聚物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号