当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A functional interplay between intein and extein sequences in protein splicing compensates for the essential block B histidine†
Chemical Science ( IF 7.6 ) Pub Date : 2018-10-03 00:00:00 , DOI: 10.1039/c8sc01074a Kristina Friedel 1, 2, 3, 4 , Monika A. Popp 4, 5, 6, 7 , Julian C. J. Matern 1, 2, 3, 4 , Emerich M. Gazdag 4, 5, 6, 7 , Ilka V. Thiel 1, 2, 3, 4 , Gerrit Volkmann 1, 2, 3, 4 , Wulf Blankenfeldt 4, 5, 6, 7, 8 , Henning D. Mootz 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2018-10-03 00:00:00 , DOI: 10.1039/c8sc01074a Kristina Friedel 1, 2, 3, 4 , Monika A. Popp 4, 5, 6, 7 , Julian C. J. Matern 1, 2, 3, 4 , Emerich M. Gazdag 4, 5, 6, 7 , Ilka V. Thiel 1, 2, 3, 4 , Gerrit Volkmann 1, 2, 3, 4 , Wulf Blankenfeldt 4, 5, 6, 7, 8 , Henning D. Mootz 1, 2, 3, 4
Affiliation
|
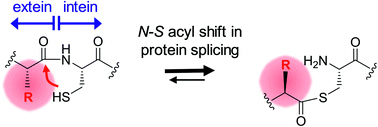
Inteins remove themselves from a precursor protein by protein splicing. Due to the concomitant structural changes of the host protein, this self-processing reaction has enabled many applications in protein biotechnology and chemical biology. We show that the evolved M86 mutant of the Ssp DnaB intein displays a significantly improved tolerance towards non-native amino acids at the N-terminally flanking (−1) extein position compared to the parent intein, in the form of both an artificially trans-splicing split intein and the cis-splicing mini-intein. Surprisingly, side chains with increased steric bulk compared to the native Gly(−1) residue, including D-amino acids, were found to compensate for the essential block B histidine in His73Ala mutants in the initial N–S acyl shift of the protein splicing pathway. In the case of the M86 intein, large (−1) side chains can even rescue protein splicing activity as a whole. With the comparison of three crystal structures, namely of the M86 intein as well as of its Gly(−1)Phe and Gly(−1)Phe/His73Ala mutants, our data supports a model in which the intein's active site can exert a strain by varying mechanisms on the different angles of the scissile bond at the extein–intein junction to effect a ground-state destabilization. The compensatory mechanism of the block B histidine is the first example for the direct functional role of an extein residue in protein splicing. It sheds new light on the extein–intein interplay and on possible consequences of their co-evolution as well as on the laboratory engineering of improved inteins.
中文翻译:

蛋白质剪接过程中内含肽和内含肽序列之间的功能性相互作用补偿了必需的嵌段B组氨酸†
内含肽通过蛋白质剪接将自身从前体蛋白质中去除。由于宿主蛋白质的结构变化,这种自我加工反应已使蛋白质生物技术和化学生物学领域有了许多应用。我们显示,与亲本intein相比,Ssp DnaB intein的进化M86突变体对N-末端侧翼(-1)extein位置的非天然氨基酸表现出显着改善的耐受性,二者均为人工反式-剪接分裂内含子和顺式剪接微型内含子。令人惊讶的是,与天然Gly(-1)残基(包括D)相比,侧链的空间体积增加了-氨基酸被发现可以补偿His73Ala突变体在蛋白质剪接途径的最初N–S酰基转移中必需的B组氨酸。对于M86内含子,大的(-1)侧链甚至可以整体上拯救蛋白剪接活性。通过比较三个晶体结构,即M86内含肽及其Gly(-1)Phe和Gly(-1)Phe / His73Ala突变体,我们的数据支持了一个模型,其中内含肽的活性位点可以施加毒株通过在外在蛋白-内在蛋白连接处的易裂键的不同角度上的不同机制来实现基态不稳定。嵌段B组氨酸的补偿机制是蛋白水解中extein残基的直接功能性作用的第一个例子。
更新日期:2018-10-03
中文翻译:

蛋白质剪接过程中内含肽和内含肽序列之间的功能性相互作用补偿了必需的嵌段B组氨酸†
内含肽通过蛋白质剪接将自身从前体蛋白质中去除。由于宿主蛋白质的结构变化,这种自我加工反应已使蛋白质生物技术和化学生物学领域有了许多应用。我们显示,与亲本intein相比,Ssp DnaB intein的进化M86突变体对N-末端侧翼(-1)extein位置的非天然氨基酸表现出显着改善的耐受性,二者均为人工反式-剪接分裂内含子和顺式剪接微型内含子。令人惊讶的是,与天然Gly(-1)残基(包括D)相比,侧链的空间体积增加了-氨基酸被发现可以补偿His73Ala突变体在蛋白质剪接途径的最初N–S酰基转移中必需的B组氨酸。对于M86内含子,大的(-1)侧链甚至可以整体上拯救蛋白剪接活性。通过比较三个晶体结构,即M86内含肽及其Gly(-1)Phe和Gly(-1)Phe / His73Ala突变体,我们的数据支持了一个模型,其中内含肽的活性位点可以施加毒株通过在外在蛋白-内在蛋白连接处的易裂键的不同角度上的不同机制来实现基态不稳定。嵌段B组氨酸的补偿机制是蛋白水解中extein残基的直接功能性作用的第一个例子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号