当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Distinct Nanostructures and Organogel Driven by Reversible Molecular Switching of a Tetraphenylethene-Involved Calix[4]arene-Based Amphiphilic [2]Rotaxane
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-10-03 00:00:00 , DOI: 10.1021/acs.chemmater.8b03286 Reguram Arumugaperumal,Putikam Raghunath,Ming-Chang Lin,Wen-Sheng Chung
Chemistry of Materials ( IF 7.2 ) Pub Date : 2018-10-03 00:00:00 , DOI: 10.1021/acs.chemmater.8b03286 Reguram Arumugaperumal,Putikam Raghunath,Ming-Chang Lin,Wen-Sheng Chung
|
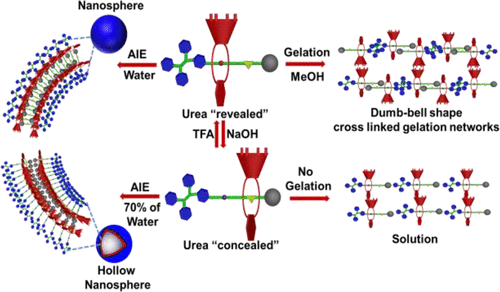
Aggregation induced emission (AIE) active and acid/base controllable amphiphilic [2]rotaxanes R1 and R2 were successfully constructed with tetraphenylethene (TPE) as a stopper and t-butylcalix[4]arene or calix[4]arene macrocycle as a wheel over the axle component. The AIE effect of [2]rotaxanes R1 and R2 was greatly affected by the molecular shuttling of t-butylcalix[4]arene or calix[4]arene macrocycle, which was triggered by the acid/base strategy. In the case of [2]rotaxane R1, aggregation was achieved in the presence of less amount of water compared with those of [2]rotaxane R2, and the deprotonated [2]rotaxanes R1-b and R2-b, owing to the stronger interaction between the TPE and t-butylcalix[4]arene macrocycle and restricted intramolecular rotation (RIR), thus making it responses in less quantity of water along with highly fluorescent emission. [2]Rotaxane R1-b started to aggregate at 70% water fraction (fw), while [2]rotaxane R2-b started to aggregate at 75% fw which allowed them to morph into hollow nanospheres, whereas they formed only nanospheres at 99% fw in CH3CN/water cosolvent system due to the higher degree of aggregation in aqueous media. To our delight, controllable morphology of self-assembled structures was indeed formed from these [2]rotaxanes. Interestingly, by the interplay of a wide range of multi-self-assembly driving forces, the slack stacking of rotaxane unit forms a hollow on the surface of nanospheres to become hollow nanospheres. Among the four [2]rotaxanes studied here, R1 possessed a narrower HOMO–LUMO band gap compared to those others, as confirmed by computational study. Furthermore, only [2]rotaxane R1 formed organogel in methanol solvent and its reversible gel–sol transition could be achieved by the addition of acid and base. This implies that the formation of dumbbell shape cross-linked 3D network structures were mainly governed by π–π stacking, van der Waals force, and intermolecular H-bonding interactions during the gelation processes.
中文翻译:

不同的纳米结构和由四苯乙烯参与的杯[4]芳烃两亲[2]轮烷的可逆分子转换驱动的有机凝胶。
以四苯乙烯(TPE)为终止剂,叔丁基杯[4]芳烃或杯[4]芳烃大环化合物为取代基,成功构建了聚集诱导发射(AIE)活性和酸/碱可控制的两亲[2]轮烷R1和R2。车轴组件。[2]轮烷R1和R2的AIE效应受叔丁基杯[4]芳烃或杯[4]芳烃大环的分子穿梭的影响,这是由酸/碱策略触发的。在[2]轮烷R1的情况下,与[2]轮烷R2和去质子化的[2]轮烷相比,在存在较少水的情况下实现了聚集。R1-b和R2-b是由于TPE与叔丁基杯[4]芳烃大环之间更强的相互作用以及受限制的分子内旋转(RIR),因此使其在较少量的水中响应,并发出高荧光。[2]轮烷R1-b开始以70%的水分数(f w)聚集,而[2]轮烷R2-b开始以75%的f w聚集,这使它们变形为空心纳米球,而它们仅形成纳米球。在99%˚F瓦特在CH 3CN /水助溶剂系统,因为在水性介质中具有较高的聚集度。令我们高兴的是,这些[2]轮烷确实形成了可控制的自组装结构形态。有趣的是,通过广泛的多自组装驱动力的相互作用,轮烷的松弛堆叠在纳米球的表面上形成了空心,从而成为空心的纳米球。计算研究证实,在本文研究的四种[2]轮烷中,R1的HOMO-LUMO带隙比其他的窄。此外,只有[2]轮烷R1在甲醇溶剂中形成有机凝胶,其可逆的凝胶-溶胶转变可通过添加酸和碱来实现。这意味着哑铃形交联的3D网络结构的形成主要受凝胶过程中的π-π堆积,范德华力和分子间H键相互作用的控制。
更新日期:2018-10-03
中文翻译:

不同的纳米结构和由四苯乙烯参与的杯[4]芳烃两亲[2]轮烷的可逆分子转换驱动的有机凝胶。
以四苯乙烯(TPE)为终止剂,叔丁基杯[4]芳烃或杯[4]芳烃大环化合物为取代基,成功构建了聚集诱导发射(AIE)活性和酸/碱可控制的两亲[2]轮烷R1和R2。车轴组件。[2]轮烷R1和R2的AIE效应受叔丁基杯[4]芳烃或杯[4]芳烃大环的分子穿梭的影响,这是由酸/碱策略触发的。在[2]轮烷R1的情况下,与[2]轮烷R2和去质子化的[2]轮烷相比,在存在较少水的情况下实现了聚集。R1-b和R2-b是由于TPE与叔丁基杯[4]芳烃大环之间更强的相互作用以及受限制的分子内旋转(RIR),因此使其在较少量的水中响应,并发出高荧光。[2]轮烷R1-b开始以70%的水分数(f w)聚集,而[2]轮烷R2-b开始以75%的f w聚集,这使它们变形为空心纳米球,而它们仅形成纳米球。在99%˚F瓦特在CH 3CN /水助溶剂系统,因为在水性介质中具有较高的聚集度。令我们高兴的是,这些[2]轮烷确实形成了可控制的自组装结构形态。有趣的是,通过广泛的多自组装驱动力的相互作用,轮烷的松弛堆叠在纳米球的表面上形成了空心,从而成为空心的纳米球。计算研究证实,在本文研究的四种[2]轮烷中,R1的HOMO-LUMO带隙比其他的窄。此外,只有[2]轮烷R1在甲醇溶剂中形成有机凝胶,其可逆的凝胶-溶胶转变可通过添加酸和碱来实现。这意味着哑铃形交联的3D网络结构的形成主要受凝胶过程中的π-π堆积,范德华力和分子间H键相互作用的控制。









































 京公网安备 11010802027423号
京公网安备 11010802027423号