当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphonate-Directed Catalytic Asymmetric Hydroboration: Delivery of Boron to the More Substituted Carbon, Leading to Chiral Tertiary Benzylic Boronic Esters
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-03 00:00:00 , DOI: 10.1021/acscatal.8b03591 Suman Chakrabarty 1 , James M. Takacs 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-03 00:00:00 , DOI: 10.1021/acscatal.8b03591 Suman Chakrabarty 1 , James M. Takacs 1
Affiliation
|
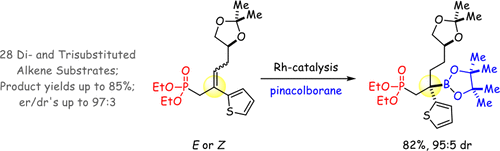
Phosphonate-directed catalytic asymmetric hydroboration (CAHB) of β-aryl/heteroaryl methylidenes and trisubstituted alkenes by pinacolborane enables facile access to functionalized, chiral tertiary benzylic boronic esters. Hydroboration is catalyzed by a chiral rhodium catalyst prepared in situ from a Rh(I) precursor in combination with a simple TADDOL-derived chiral cyclic monophosphite in a 1:1 ratio. The regio- and stereochemistry arise from the combined effects of the relative disposition of the directing group to the alkene, the alkene substitution pattern, and the necessity of an aryl substituent attached to the alkene. A range of aryl and heteroaryl substituents can be accommodated, and for several chiral substrates, the reactions are efficiently catalyst-controlled, enabling the choice of diastereomeric products as desired. Stereospecific transformations of the chiral boronic ester afford chiral phosphonates bearing a quaternary carbon stereocenter. The synthetic utility of the products is further demonstrated by α-oxidation of the phosphonate, leading to hydroxy- and oxophosphonates; the latter readily undergo elimination/substitution reactions to unmask the phosphonate functionality with the formation of aldehydes, alcohols, esters, amides, acids, and ketones.
中文翻译:

膦酸酯类催化的不对称硼氢化反应:将硼传递到更多取代的碳上,导致手性叔苄基硼酸酯
频哪醇硼烷的直接作用于β-芳基/杂芳基亚甲基和三取代烯烃的膦酸酯催化的催化不对称硼氢化反应(CAHB)使得可以轻松获得官能化的手性叔苄基硼酸酯。由Rh(I)前体与简单的TADDOL衍生的手性环状单亚磷酸酯以1:1的比例混合就地制备的手性铑催化剂催化了硼氢化反应。区域化学和立体化学是由导向基团相对于烯烃的相对位置,烯烃的取代方式以及连接到烯烃上的芳基取代基的必要性的综合作用引起的。可以容纳一定范围的芳基和杂芳基取代基,对于几种手性底物,反应可以有效地进行催化剂控制,从而可以根据需要选择非对映体产物。手性硼酸酯的立体特异性转化提供带有季碳立体中心的手性膦酸酯。通过膦酸酯的α-氧化反应进一步生成产物的合成效用,从而生成羟基膦酸酯和氧代膦酸酯;后者容易发生消除/取代反应,从而通过形成醛,醇,酯,酰胺,酸和酮来掩盖膦酸酯官能团。
更新日期:2018-10-03
中文翻译:

膦酸酯类催化的不对称硼氢化反应:将硼传递到更多取代的碳上,导致手性叔苄基硼酸酯
频哪醇硼烷的直接作用于β-芳基/杂芳基亚甲基和三取代烯烃的膦酸酯催化的催化不对称硼氢化反应(CAHB)使得可以轻松获得官能化的手性叔苄基硼酸酯。由Rh(I)前体与简单的TADDOL衍生的手性环状单亚磷酸酯以1:1的比例混合就地制备的手性铑催化剂催化了硼氢化反应。区域化学和立体化学是由导向基团相对于烯烃的相对位置,烯烃的取代方式以及连接到烯烃上的芳基取代基的必要性的综合作用引起的。可以容纳一定范围的芳基和杂芳基取代基,对于几种手性底物,反应可以有效地进行催化剂控制,从而可以根据需要选择非对映体产物。手性硼酸酯的立体特异性转化提供带有季碳立体中心的手性膦酸酯。通过膦酸酯的α-氧化反应进一步生成产物的合成效用,从而生成羟基膦酸酯和氧代膦酸酯;后者容易发生消除/取代反应,从而通过形成醛,醇,酯,酰胺,酸和酮来掩盖膦酸酯官能团。











































 京公网安备 11010802027423号
京公网安备 11010802027423号