Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-10-04 , DOI: 10.1016/j.tetlet.2018.10.005 Sambasivarao Kotha , Rashid Ali , Nageswara Rao Panguluri , Anindya Datta , Krishna K. Kannaujiya
|
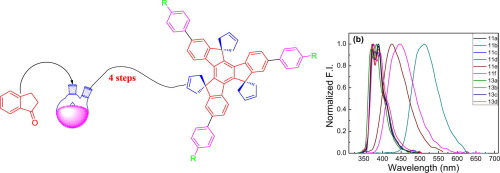
Here, we report a simple and useful strategy for a series of C3-symmetric π-conjugated and highly soluble spiro-annulated truxene derivatives. The role of substituent at C5, C10, C15, C2, C7, and C12 positions was studied. We observed that truxenes containing carbonyl groups showed red shift in the absorption maxima in comparison with compounds devoid of carbonyl group. Enhanced conjugation in these systems may be responsible for this red shift. It was observed that truxene derivatives with carbonyl moiety exhibited faster decay than other derivatives. Benzyl groups play some role in the excited state dynamics as decay becomes slower when the spiro moiety was replaced by benzyl group at C5, C10, and C15 positions. Additionally, we noticed the highest quantum yield in t-butanol solvent and least in acetonitrile.
中文翻译:

星形发射π-共轭螺螺旋体的蓝绿色星形化合物的合成及其光物理性质
在这里,我们报告了一系列简单且有用的策略,用于一系列C 3对称的π-共轭和高度可溶性的螺环化的富烯衍生物。研究了取代基在C5,C10,C15,C2,C7和C12位置上的作用。我们观察到,与不含羰基的化合物相比,含羰基的戊二烯在吸收最大值处出现红移。在这些系统中增强的共轭可能是造成这种红移的原因。观察到具有羰基部分的戊二烯衍生物表现出比其他衍生物更快的衰变。当螺环部分在C5,C10和C15位置被苄基取代时,苄基在激发态动力学中起一定作用,因为衰变变慢。此外,我们注意到,t中的量子产率最高-丁醇溶剂,至少在乙腈中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号