Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Handover mechanism of the growing pilus by the bacterial outer-membrane usher FimD
Nature ( IF 50.5 ) Pub Date : 2018-10-01 , DOI: 10.1038/s41586-018-0587-z Minge Du , Zuanning Yuan , Hongjun Yu , Nadine Henderson , Samema Sarowar , Gongpu Zhao , Glenn T. Werneburg , David G. Thanassi , Huilin Li
Nature ( IF 50.5 ) Pub Date : 2018-10-01 , DOI: 10.1038/s41586-018-0587-z Minge Du , Zuanning Yuan , Hongjun Yu , Nadine Henderson , Samema Sarowar , Gongpu Zhao , Glenn T. Werneburg , David G. Thanassi , Huilin Li
|
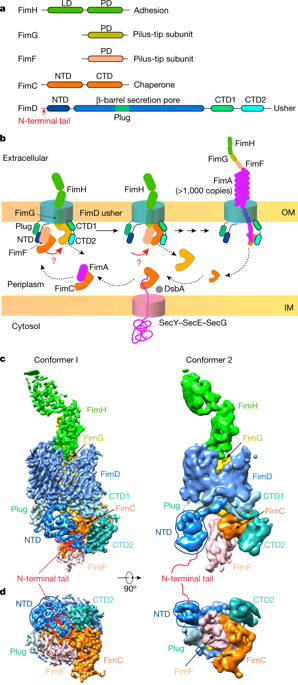
Pathogenic bacteria such as Escherichia coli assemble surface structures termed pili, or fimbriae, to mediate binding to host-cell receptors1. Type 1 pili are assembled via the conserved chaperone–usher pathway2–5. The outer-membrane usher FimD recruits pilus subunits bound by the chaperone FimC via the periplasmic N-terminal domain of the usher. Subunit translocation through the β-barrel channel of the usher occurs at the two C-terminal domains (which we label CTD1 and CTD2) of this protein. How the chaperone–subunit complex bound to the N-terminal domain is handed over to the C-terminal domains, as well as the timing of subunit polymerization into the growing pilus, have previously been unclear. Here we use cryo-electron microscopy to capture a pilus assembly intermediate (FimD–FimC–FimF–FimG–FimH) in a conformation in which FimD is in the process of handing over the chaperone-bound end of the growing pilus to the C-terminal domains. In this structure, FimF has already polymerized with FimG, and the N-terminal domain of FimD swings over to bind CTD2; the N-terminal domain maintains contact with FimC–FimF, while at the same time permitting access to the C-terminal domains. FimD has an intrinsically disordered N-terminal tail that precedes the N-terminal domain. This N-terminal tail folds into a helical motif upon recruiting the FimC-subunit complex, but reorganizes into a loop to bind CTD2 during handover. Because both the N-terminal and C-terminal domains of FimD are bound to the end of the growing pilus, the structure further suggests a mechanism for stabilizing the assembly intermediate to prevent the pilus fibre diffusing away during the incorporation of thousands of subunits.The structure of a pilus assembly intermediate reveals the timing of subunit polymerization and how chaperone–subunit complexes are transferred from N-terminal to C-terminal domains of the usher in the formation of bacterial pili.
中文翻译:

细菌外膜引导者 FimD 对生长菌毛的切换机制
大肠杆菌等致病细菌组装称为菌毛或菌毛的表面结构,以介导与宿主细胞受体的结合。1 型菌毛通过保守的分子伴侣-引导通路 2-5 组装。外膜引导者 FimD 通过引导者的周质 N 末端域招募由伴侣 FimC 结合的菌毛亚基。通过引导者的 β 桶通道的亚基易位发生在该蛋白质的两个 C 端结构域(我们标记为 CTD1 和 CTD2)。与 N 端结构域结合的分子伴侣 - 亚基复合物如何转移到 C 端结构域,以及亚基聚合到生长菌毛中的时间,以前一直不清楚。在这里,我们使用冷冻电子显微镜来捕获处于一种构象的菌毛组装中间体(FimD-FimC-FimF-FimG-FimH),其中 FimD 正在将生长菌毛的伴侣结合端移交给 C-终端域。在这个结构中,FimF 已经与 FimG 聚合,FimD 的 N 端结构域翻转过来结合 CTD2;N 端域与 FimC-FimF 保持联系,同时允许访问 C 端域。FimD 在 N 端域之前有一个本质上无序的 N 端尾部。在募集 FimC 亚基复合物时,该 N 端尾部折叠成螺旋基序,但在交接期间重组为环以结合 CTD2。因为 FimD 的 N 端和 C 端结构域都与生长菌毛的末端结合,
更新日期:2018-10-01
中文翻译:

细菌外膜引导者 FimD 对生长菌毛的切换机制
大肠杆菌等致病细菌组装称为菌毛或菌毛的表面结构,以介导与宿主细胞受体的结合。1 型菌毛通过保守的分子伴侣-引导通路 2-5 组装。外膜引导者 FimD 通过引导者的周质 N 末端域招募由伴侣 FimC 结合的菌毛亚基。通过引导者的 β 桶通道的亚基易位发生在该蛋白质的两个 C 端结构域(我们标记为 CTD1 和 CTD2)。与 N 端结构域结合的分子伴侣 - 亚基复合物如何转移到 C 端结构域,以及亚基聚合到生长菌毛中的时间,以前一直不清楚。在这里,我们使用冷冻电子显微镜来捕获处于一种构象的菌毛组装中间体(FimD-FimC-FimF-FimG-FimH),其中 FimD 正在将生长菌毛的伴侣结合端移交给 C-终端域。在这个结构中,FimF 已经与 FimG 聚合,FimD 的 N 端结构域翻转过来结合 CTD2;N 端域与 FimC-FimF 保持联系,同时允许访问 C 端域。FimD 在 N 端域之前有一个本质上无序的 N 端尾部。在募集 FimC 亚基复合物时,该 N 端尾部折叠成螺旋基序,但在交接期间重组为环以结合 CTD2。因为 FimD 的 N 端和 C 端结构域都与生长菌毛的末端结合,











































 京公网安备 11010802027423号
京公网安备 11010802027423号