当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The electrochemical reduction kinetics of oxygen in dimethylsulfoxide
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2018-11-01 , DOI: 10.1016/j.jelechem.2018.09.052 Ruba Hendi , Peter H. Robbs , Beatrice Sampson , Ryan Pearce , Neil V. Rees
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2018-11-01 , DOI: 10.1016/j.jelechem.2018.09.052 Ruba Hendi , Peter H. Robbs , Beatrice Sampson , Ryan Pearce , Neil V. Rees
|
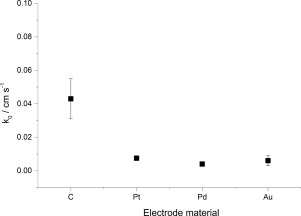
Abstract The quasi-reversible one-electron reduction of oxygen in dimethylsulfoxide is reported for a range of electrode materials (C, Pt, Pd, and Au) and temperatures (293–343 K). Modelling was undertaken using Butler-Volmer and symmetric Marcus-Hush methods, with the former found to provide more reproducible results for this system, in agreement with previous reports of quasi-reversible systems. The reorganisation energy for the reaction was found to be ca. 1.0 eV, and the reaction confirmed to be predominantly outer-sphere. The observed standard electrochemical rate constant (k0) is ca. 5.7 times faster for C electrodes than Pt, despite having a lower electronic density of states.
中文翻译:

二甲亚砜中氧的电化学还原动力学
摘要 据报道,在一系列电极材料(C、Pt、Pd 和 Au)和温度(293-343 K)下,二甲亚砜中氧的准可逆单电子还原。建模是使用 Butler-Volmer 和对称 Marcus-Hush 方法进行的,发现前者为该系统提供了更多可重复的结果,与之前关于准可逆系统的报告一致。发现反应的重组能约为。1.0 eV,并且确认反应主要是外球体。观察到的标准电化学速率常数 (k0) 约为。尽管具有较低的电子态密度,但 C 电极比 Pt 快 5.7 倍。
更新日期:2018-11-01
中文翻译:

二甲亚砜中氧的电化学还原动力学
摘要 据报道,在一系列电极材料(C、Pt、Pd 和 Au)和温度(293-343 K)下,二甲亚砜中氧的准可逆单电子还原。建模是使用 Butler-Volmer 和对称 Marcus-Hush 方法进行的,发现前者为该系统提供了更多可重复的结果,与之前关于准可逆系统的报告一致。发现反应的重组能约为。1.0 eV,并且确认反应主要是外球体。观察到的标准电化学速率常数 (k0) 约为。尽管具有较低的电子态密度,但 C 电极比 Pt 快 5.7 倍。









































 京公网安备 11010802027423号
京公网安备 11010802027423号