当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Highly Bioactive Lys-Deficient IFN Leads to a Site-Specific Di-PEGylated IFN with Equivalent Bioactivity to That of Unmodified IFN-α2b
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2018-10-02 00:00:00 , DOI: 10.1021/acssynbio.8b00188 Takashi Imada , Koji Moriya , Masahiko Uchiyama , Naoto Inukai , Mitsuhiro Hitotsuyanagi , Akiko Masuda 1 , Takehiro Suzuki 1 , Shotaro Ayukawa 2 , Yo-ichi Tagawa , Naoshi Dohmae 1 , Michinori Kohara 3 , Masayuki Yamamura , Daisuke Kiga 2
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2018-10-02 00:00:00 , DOI: 10.1021/acssynbio.8b00188 Takashi Imada , Koji Moriya , Masahiko Uchiyama , Naoto Inukai , Mitsuhiro Hitotsuyanagi , Akiko Masuda 1 , Takehiro Suzuki 1 , Shotaro Ayukawa 2 , Yo-ichi Tagawa , Naoshi Dohmae 1 , Michinori Kohara 3 , Masayuki Yamamura , Daisuke Kiga 2
Affiliation
|
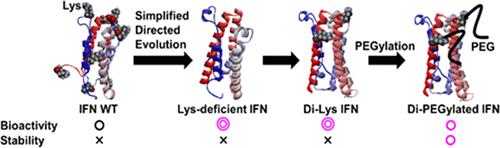
Although conjugation with polyethylene glycol (PEGylation) improves the pharmacokinetics of therapeutic proteins, it drastically decreases their bioactivity. Site-specific PEGylation counters the reduction in bioactivity, but developing PEGylated proteins with equivalent bioactivity to that of their unmodified counterparts remains challenging. This study aimed to generate PEGylated proteins with equivalent bioactivity to that of unmodified counterparts. Using interferon (IFN) as a model protein, a highly bioactive Lys-deficient protein variant generated using our unique directed evolution methods enables the design of a site-specific di-PEGylated protein. Antiviral activity of our di-PEGylated IFN was similar to that of unmodified IFN-α2b. The di-PEGylated IFN exhibited 3.0-fold greater antiviral activity than that of a commercial PEGylated IFN. Moreover, our di-PEGylated IFN showed higher in vitro and in vivo stability than those of unmodified IFN-α2b. Hence, we propose that highly bioactive Lys-deficient proteins solve the limitation of conventional PEGylation with respect to the reduction in bioactivity of PEGylated proteins.
中文翻译:

高生物活性的Lys缺陷型IFN导致位点特异性二聚乙二醇化IFN具有与未修饰的IFN-α2b相当的生物活性
尽管与聚乙二醇结合(聚乙二醇化)可以改善治疗性蛋白质的药代动力学,但会大大降低其生物活性。位点特异性PEG化可以抵消生物活性的降低,但是开发具有与未修饰的同等生物活性相当的生物活性的PEG化蛋白质仍然具有挑战性。这项研究旨在产生与未修饰的对应物具有同等生物活性的聚乙二醇化蛋白。使用干扰素(IFN)作为模型蛋白,使用我们独特的定向进化方法生成的具有高生物活性的Lys缺陷型蛋白变体可以设计位点特异性二聚乙二醇化蛋白。我们的二聚乙二醇化IFN的抗病毒活性与未修饰的IFN-α2b相似。与商用PEG化IFN相比,双PEG化IFN的抗病毒活性高3.0倍。体外和体内的稳定性要高于未修饰的IFN-α2b。因此,我们提出高生物活性的Lys缺陷蛋白解决了传统的PEG化的局限性,即降低了PEG化蛋白的生物活性。
更新日期:2018-10-02
中文翻译:

高生物活性的Lys缺陷型IFN导致位点特异性二聚乙二醇化IFN具有与未修饰的IFN-α2b相当的生物活性
尽管与聚乙二醇结合(聚乙二醇化)可以改善治疗性蛋白质的药代动力学,但会大大降低其生物活性。位点特异性PEG化可以抵消生物活性的降低,但是开发具有与未修饰的同等生物活性相当的生物活性的PEG化蛋白质仍然具有挑战性。这项研究旨在产生与未修饰的对应物具有同等生物活性的聚乙二醇化蛋白。使用干扰素(IFN)作为模型蛋白,使用我们独特的定向进化方法生成的具有高生物活性的Lys缺陷型蛋白变体可以设计位点特异性二聚乙二醇化蛋白。我们的二聚乙二醇化IFN的抗病毒活性与未修饰的IFN-α2b相似。与商用PEG化IFN相比,双PEG化IFN的抗病毒活性高3.0倍。体外和体内的稳定性要高于未修饰的IFN-α2b。因此,我们提出高生物活性的Lys缺陷蛋白解决了传统的PEG化的局限性,即降低了PEG化蛋白的生物活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号