当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Friedel–Crafts strategy for preparing 3-acylindoles
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-10-03 , DOI: 10.1039/c8ob02094a Lian-Hua Li 1, 2, 3, 4, 5 , Zhi-Jie Niu 1, 2, 3, 4, 5 , Yong-Min Liang 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-10-03 , DOI: 10.1039/c8ob02094a Lian-Hua Li 1, 2, 3, 4, 5 , Zhi-Jie Niu 1, 2, 3, 4, 5 , Yong-Min Liang 1, 2, 3, 4, 5
Affiliation
|
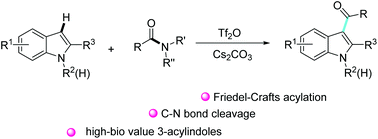
A selective Friedel–Crafts acylation of indoles via an unusual cleavage of the amide C–N bond was achieved by triflic anhydride activation. This method offers rapid efficient access to high-biological-value 3-acylindoles, performs a series of scrupulous mechanistic studies and offers a strong courage that amide synthons can form new C–C bonds under transition-metal-free conditions.
中文翻译:

制备3-acylindoles的新Friedel-Crafts策略
吲哚的选择性Friedel-Crafts酰化经由酰胺C-N键的一个不寻常的裂解物通过三氟甲磺酸酐的活化来实现。这种方法可以快速有效地获得具有高生物价值的3-acylindoles,进行了一系列严格的机理研究,并提供了强大的勇气,即酰胺合成子可以在无过渡金属的条件下形成新的C-C键。
更新日期:2018-11-02
中文翻译:

制备3-acylindoles的新Friedel-Crafts策略
吲哚的选择性Friedel-Crafts酰化经由酰胺C-N键的一个不寻常的裂解物通过三氟甲磺酸酐的活化来实现。这种方法可以快速有效地获得具有高生物价值的3-acylindoles,进行了一系列严格的机理研究,并提供了强大的勇气,即酰胺合成子可以在无过渡金属的条件下形成新的C-C键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号