当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanostructured multi-block copolymer single-ion conductors for safer high-performance lithium batteries†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-10-03 00:00:00 , DOI: 10.1039/c8ee02093k Huu-Dat Nguyen 1, 2, 3, 4, 5 , Guk-Tae Kim 6, 7, 8, 9, 10 , Junli Shi 8, 11, 12, 13, 14 , Elie Paillard 8, 11, 12, 13 , Patrick Judeinstein 15, 16, 17, 18, 19 , Sandrine Lyonnard 1, 2, 5, 20, 21 , Dominic Bresser 6, 7, 8, 9, 10 , Cristina Iojoiu 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-10-03 00:00:00 , DOI: 10.1039/c8ee02093k Huu-Dat Nguyen 1, 2, 3, 4, 5 , Guk-Tae Kim 6, 7, 8, 9, 10 , Junli Shi 8, 11, 12, 13, 14 , Elie Paillard 8, 11, 12, 13 , Patrick Judeinstein 15, 16, 17, 18, 19 , Sandrine Lyonnard 1, 2, 5, 20, 21 , Dominic Bresser 6, 7, 8, 9, 10 , Cristina Iojoiu 1, 2, 3, 4, 5
Affiliation
|
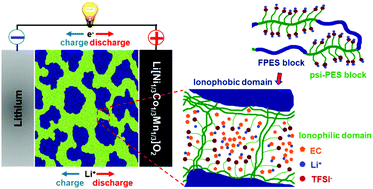
The greatest challenges towards the worldwide success of battery-powered electric vehicles revolve around the safety and energy density of the battery. Single-ion conducting polymer electrolytes address both challenges by replacing the flammable and unstable liquid electrolytes and enabling dendrite-free cycling of high-energy lithium metal anodes. To date, however, their commercial use has been hindered by insufficient ionic conductivities at ambient temperature (commonly not exceeding 10−6 S cm−1) and the limited electrochemical stability towards oxidation, in particular when incorporating ether-type building blocks, limiting their application to rather low-voltage cathode materials like LiFePO4. Here, we introduce ether-free, nanostructured multi-block copolymers as single-ion conducting electrolytes, providing high thermal stability and self-extinguishing properties and, if plasticized with ethylene carbonate, ionic conductivities exceeding 10−3 S cm−1 above 30 °C, i.e., approaching that of state-of-the-art liquid electrolytes. Moreover, these single-ion conducting ionomers present highly reversible lithium cycling for more than 1000 h and, as a result of their excellent electrochemical stability, highly stable cycling of Li[Ni1/3Co1/3Mn1/3]O2 cathodes. To the best of our knowledge, this is the first polymer electrolyte that presents such remarkable ionic conductivity and outstanding electrochemical stability towards both reduction and oxidation, thus, paving the way for advanced high-energy lithium metal batteries. Remarkably, the realization of well-defined continuous ionic domains appears to be the key to efficient charge transport through the electrolyte bulk and across the electrode/electrolyte interface, highlighting the importance of the self-assembling nanostructure. The latter is achieved by carefully (i) designing the copolymer structure, i.e., introducing alternating ionic blocks with a very regular distribution of weakly coordinating anions along the polymer chain and rigid blocks, which are completely immiscible with ethylene carbonate, and (ii) choosing the processing solvent, taking into account its interaction with the different copolymer blocks.
中文翻译:

纳米结构多嵌段共聚物单离子导体,可提供更安全的高性能锂电池†
电动汽车在全球范围内取得成功的最大挑战在于电池的安全性和能量密度。单离子导电聚合物电解质通过替代易燃和不稳定的液体电解质并实现高能锂金属阳极的无枝晶循环而解决了这两个挑战。然而,迄今为止,由于在环境温度下离子电导率不足(通常不超过10 -6 S cm -1)和对氧化的有限电化学稳定性(特别是在掺入醚型结构单元时),阻碍了它们的商业应用。应用于相当低电压的正极材料,如LiFePO 4。在这里,我们引入无醚,纳米结构的多嵌段共聚物作为单离子导电电解质,提供高的热稳定性和自熄性,并且如果用碳酸亚乙酯增塑,则在30°以上时离子电导率将超过10 -3 S cm -1 C,即接近最先进的液体电解质的温度。此外,这些单离子导电离聚物具有高度可逆的锂循环超过1000小时,并且由于其出色的电化学稳定性,Li [Ni 1/3 Co 1/3 Mn 1/3 ] O 2具有高度稳定的循环阴极。据我们所知,这是第一款具有如此出色的离子电导率和出色的还原和氧化电化学稳定性的聚合物电解质,从而为高级高能锂金属电池铺平了道路。值得注意的是,明确定义的连续离子域的实现似乎是有效电荷通过电解质主体以及跨电极/电解质界面有效传输的关键,突出了自组装纳米结构的重要性。后者是通过仔细地(i)设计共聚物结构来实现的,即引入了交替的离子嵌段,这些嵌段沿着聚合物链和刚性嵌段非常规则地分布着弱配位阴离子,而刚性嵌段与碳酸亚乙酯是完全不相溶的;(ii)考虑到加工溶剂与不同共聚物嵌段的相互作用,选择加工溶剂。
更新日期:2018-10-03
中文翻译:

纳米结构多嵌段共聚物单离子导体,可提供更安全的高性能锂电池†
电动汽车在全球范围内取得成功的最大挑战在于电池的安全性和能量密度。单离子导电聚合物电解质通过替代易燃和不稳定的液体电解质并实现高能锂金属阳极的无枝晶循环而解决了这两个挑战。然而,迄今为止,由于在环境温度下离子电导率不足(通常不超过10 -6 S cm -1)和对氧化的有限电化学稳定性(特别是在掺入醚型结构单元时),阻碍了它们的商业应用。应用于相当低电压的正极材料,如LiFePO 4。在这里,我们引入无醚,纳米结构的多嵌段共聚物作为单离子导电电解质,提供高的热稳定性和自熄性,并且如果用碳酸亚乙酯增塑,则在30°以上时离子电导率将超过10 -3 S cm -1 C,即接近最先进的液体电解质的温度。此外,这些单离子导电离聚物具有高度可逆的锂循环超过1000小时,并且由于其出色的电化学稳定性,Li [Ni 1/3 Co 1/3 Mn 1/3 ] O 2具有高度稳定的循环阴极。据我们所知,这是第一款具有如此出色的离子电导率和出色的还原和氧化电化学稳定性的聚合物电解质,从而为高级高能锂金属电池铺平了道路。值得注意的是,明确定义的连续离子域的实现似乎是有效电荷通过电解质主体以及跨电极/电解质界面有效传输的关键,突出了自组装纳米结构的重要性。后者是通过仔细地(i)设计共聚物结构来实现的,即引入了交替的离子嵌段,这些嵌段沿着聚合物链和刚性嵌段非常规则地分布着弱配位阴离子,而刚性嵌段与碳酸亚乙酯是完全不相溶的;(ii)考虑到加工溶剂与不同共聚物嵌段的相互作用,选择加工溶剂。










































 京公网安备 11010802027423号
京公网安备 11010802027423号