当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A reaction–diffusion kinetic model for the heterogeneous N-deacetylation step in chitin material conversion to chitosan in catalytic alkaline solutions
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-10-02 00:00:00 , DOI: 10.1039/c8re00170g Bojana Bradić 1, 2, 3 , David Bajec 1, 2, 3 , Andrej Pohar 1, 2, 3 , Uroš Novak 1, 2, 3 , Blaž Likozar 1, 2, 3
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-10-02 00:00:00 , DOI: 10.1039/c8re00170g Bojana Bradić 1, 2, 3 , David Bajec 1, 2, 3 , Andrej Pohar 1, 2, 3 , Uroš Novak 1, 2, 3 , Blaž Likozar 1, 2, 3
Affiliation
|
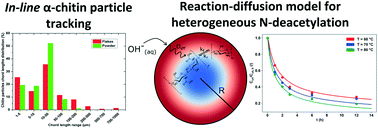
This conversion study provides a new mechanistic insight into the modelling of the heterogeneous N-deacetylation step of α-chitin, obtained from waste crustacean shells, using a catalytic alkaline solution at different operating temperatures (25–80 °C) and concentrations (50–80 wt%). Transient particle-size distributions for two separate experiments with smaller powder or larger flake morphologies were obtained by applying an inline tracking system. The degree of deacetylation (DDA), polymer molecular mass and viscosity of the deacetylated raw resource were continuously monitored with time until the maximum DDA was reached. The mechanism of the conversion of an average biopolymer chain was described with a reaction–diffusion kinetic model, solved for all fraction intervals, and optimised. The effective diffusivity coefficient was estimated with regression analysis; the geometry of the particles was approximated as having spherical dimensions, and material isotropy was presumed. A second-order reaction process took place, since the content of the dissolved hydroxyl ions inside the pores was not considered constant. Additionally, several analytical tools such as scanning electron microscopy (SEM) was employed as well as specific porosity measurements to get a deeper phenomenological understanding of the material's morphological transformation. The selected mathematical relationship granted a relatively good agreement for the cumulative experimental data by regarding the kinetics in the initial consumption phase, as well as the subsequent transport resistance for OH−. The developed descriptive approach, together with online measuring methods, could enable a more comprehensive option for commercial productivity increase, as well as unit operations scale-up.
中文翻译:

催化碱性溶液中几丁质材料转化为壳聚糖的非均相N-脱乙酰化步骤的反应扩散动力学模型
这项转化研究为在不同工作温度(25–80°C)和浓度(50–50°C)下使用催化碱性溶液从废甲壳类动物壳中获得的α-甲壳质的异质N-脱乙酰化步骤的建模提供了新的力学见解。 80重量%)。通过应用在线跟踪系统,可以得到两个较小粉末或较大薄片形态的单独实验的瞬态粒度分布。随时间连续监测脱乙酰化程度(DDA),聚合物分子量和脱乙酰化原料的粘度,直到达到最大DDA。用反应扩散动力学模型描述了平均生物聚合物链转化的机理,求解了所有馏分间隔并进行了优化。有效扩散系数通过回归分析估算;颗粒的几何形状近似为具有球形尺寸,并推测出材料各向同性。由于孔内溶解的氢氧根离子的含量不恒定,因此发生了二级反应过程。此外,还使用了多种分析工具,例如扫描电子显微镜(SEM)以及特定的孔隙率测量,以对材料的形态转变获得更深刻的现象学理解。通过考虑初始消耗阶段的动力学以及随后的OH传输阻力,所选的数学关系为累积实验数据提供了相对较好的一致性 颗粒的几何形状近似为具有球形尺寸,并推测出材料各向同性。由于孔内溶解的氢氧根离子的含量不恒定,因此发生了二级反应过程。此外,还使用了多种分析工具,例如扫描电子显微镜(SEM)以及特定的孔隙率测量,以对材料的形态转变获得更深刻的现象学理解。通过考虑初始消耗阶段的动力学以及随后的OH传输阻力,所选的数学关系为累积实验数据提供了相对较好的一致性 颗粒的几何形状近似为具有球形尺寸,并推测出材料各向同性。发生了二级反应过程,因为孔中溶解的氢氧根离子的含量不被认为是恒定的。此外,还使用了多种分析工具,例如扫描电子显微镜(SEM)以及特定的孔隙率测量,以对材料的形态转变获得更深刻的现象学理解。通过考虑初始消耗阶段的动力学以及随后的OH传输阻力,所选的数学关系为累积实验数据提供了相对较好的一致性 因为孔内溶解的氢氧根离子的含量不被认为是恒定的。此外,还使用了多种分析工具,例如扫描电子显微镜(SEM)以及特定的孔隙率测量,以对材料的形态转变获得更深刻的现象学理解。通过考虑初始消耗阶段的动力学以及随后的OH传输阻力,所选的数学关系为累积实验数据提供了相对较好的一致性 因为孔内溶解的氢氧根离子的含量不被认为是恒定的。此外,还使用了多种分析工具,例如扫描电子显微镜(SEM)以及特定的孔隙率测量,以对材料的形态转变获得更深刻的现象学理解。通过考虑初始消耗阶段的动力学以及随后的OH传输阻力,所选的数学关系为累积实验数据提供了相对较好的一致性-。发达的描述性方法以及在线测量方法可以为提高商业生产率以及扩大单位运营规模提供更全面的选择。
更新日期:2018-10-02
中文翻译:

催化碱性溶液中几丁质材料转化为壳聚糖的非均相N-脱乙酰化步骤的反应扩散动力学模型
这项转化研究为在不同工作温度(25–80°C)和浓度(50–50°C)下使用催化碱性溶液从废甲壳类动物壳中获得的α-甲壳质的异质N-脱乙酰化步骤的建模提供了新的力学见解。 80重量%)。通过应用在线跟踪系统,可以得到两个较小粉末或较大薄片形态的单独实验的瞬态粒度分布。随时间连续监测脱乙酰化程度(DDA),聚合物分子量和脱乙酰化原料的粘度,直到达到最大DDA。用反应扩散动力学模型描述了平均生物聚合物链转化的机理,求解了所有馏分间隔并进行了优化。有效扩散系数通过回归分析估算;颗粒的几何形状近似为具有球形尺寸,并推测出材料各向同性。由于孔内溶解的氢氧根离子的含量不恒定,因此发生了二级反应过程。此外,还使用了多种分析工具,例如扫描电子显微镜(SEM)以及特定的孔隙率测量,以对材料的形态转变获得更深刻的现象学理解。通过考虑初始消耗阶段的动力学以及随后的OH传输阻力,所选的数学关系为累积实验数据提供了相对较好的一致性 颗粒的几何形状近似为具有球形尺寸,并推测出材料各向同性。由于孔内溶解的氢氧根离子的含量不恒定,因此发生了二级反应过程。此外,还使用了多种分析工具,例如扫描电子显微镜(SEM)以及特定的孔隙率测量,以对材料的形态转变获得更深刻的现象学理解。通过考虑初始消耗阶段的动力学以及随后的OH传输阻力,所选的数学关系为累积实验数据提供了相对较好的一致性 颗粒的几何形状近似为具有球形尺寸,并推测出材料各向同性。发生了二级反应过程,因为孔中溶解的氢氧根离子的含量不被认为是恒定的。此外,还使用了多种分析工具,例如扫描电子显微镜(SEM)以及特定的孔隙率测量,以对材料的形态转变获得更深刻的现象学理解。通过考虑初始消耗阶段的动力学以及随后的OH传输阻力,所选的数学关系为累积实验数据提供了相对较好的一致性 因为孔内溶解的氢氧根离子的含量不被认为是恒定的。此外,还使用了多种分析工具,例如扫描电子显微镜(SEM)以及特定的孔隙率测量,以对材料的形态转变获得更深刻的现象学理解。通过考虑初始消耗阶段的动力学以及随后的OH传输阻力,所选的数学关系为累积实验数据提供了相对较好的一致性 因为孔内溶解的氢氧根离子的含量不被认为是恒定的。此外,还使用了多种分析工具,例如扫描电子显微镜(SEM)以及特定的孔隙率测量,以对材料的形态转变获得更深刻的现象学理解。通过考虑初始消耗阶段的动力学以及随后的OH传输阻力,所选的数学关系为累积实验数据提供了相对较好的一致性-。发达的描述性方法以及在线测量方法可以为提高商业生产率以及扩大单位运营规模提供更全面的选择。











































 京公网安备 11010802027423号
京公网安备 11010802027423号