当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Silver(I)-Catalyzed C–X, C–C, C–N, and C–O Cross-Couplings Using Aminoquinoline Directing Group via Elusive Aryl-Ag(III) Species
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-10-01 00:00:00 , DOI: 10.1021/acscatal.8b03257 Lorena Capdevila 1 , Erik Andris 2 , Anamarija Briš 2, 3 , Màrius Tarrés 1 , Steven Roldán-Gómez 1 , Jana Roithová 2, 4 , Xavi Ribas 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-10-01 00:00:00 , DOI: 10.1021/acscatal.8b03257 Lorena Capdevila 1 , Erik Andris 2 , Anamarija Briš 2, 3 , Màrius Tarrés 1 , Steven Roldán-Gómez 1 , Jana Roithová 2, 4 , Xavi Ribas 1
Affiliation
|
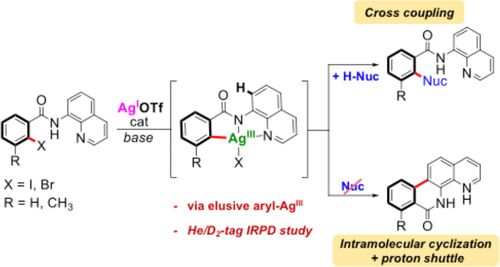
Cross-coupling transformations are a powerful tool in organic synthesis. It is known that this kind of transformation undergoes 2-electron redox processes, and, for this reason, silver has been nearly forgotten as catalyst for cross-couplings because silver is mainly considered as a 1-electron redox metal. Herein, we disclose effective Ag(I)-catalyzed cross-coupling transformations using bidentate aminoquinoline as a directing group toward different nucleophiles to form C–C, C–N, and C–O bonds. DFT calculations indicate the feasible oxidative addition of L1-I substrate via the Ag(I)/Ag(III) catalytic cycle. Furthermore, ion spectroscopy experiments suggest a highly reactive aryl-Ag(III) that in the absence of nucleophiles reacts to form an intermolecular cyclic product [5d-Ag(I)-CH3CN], which in solution forms 5a. This work proves that silver can undergo 2-electron redox processes in cross-coupling reactions like Pd and Cu.
中文翻译:

银(I)催化的CX,CC,CN和C-O交叉偶联,使用氨基喹啉直接基团通过难以捉摸的芳基-Ag(III)物种进行
交叉耦合转换是有机合成中的强大工具。众所周知,这种转变经历了2-电子氧化还原过程,并且由于这个原因,因为银主要被认为是1-电子氧化还原金属,所以几乎已经忘记了银作为交叉偶联的催化剂。在这里,我们公开了有效的Ag(I)催化的交叉偶联转化,使用双齿氨基喹啉作为指向不同亲核试剂的直链基团,形成C–C,C–N和C–O键。DFT计算表明通过Ag(I)/ Ag(III)催化循环可实现L 1 -I底物的氧化加成。此外,离子光谱实验表明,在没有亲核试剂的情况下,高反应性的芳基-Ag(III)会反应形成分子间环状产物[5d-Ag(I)-CH 3 CN],其在溶液中形成5a。这项工作证明银可以在Pd和Cu等交叉偶联反应中经历2-电子氧化还原过程。
更新日期:2018-10-01
中文翻译:

银(I)催化的CX,CC,CN和C-O交叉偶联,使用氨基喹啉直接基团通过难以捉摸的芳基-Ag(III)物种进行
交叉耦合转换是有机合成中的强大工具。众所周知,这种转变经历了2-电子氧化还原过程,并且由于这个原因,因为银主要被认为是1-电子氧化还原金属,所以几乎已经忘记了银作为交叉偶联的催化剂。在这里,我们公开了有效的Ag(I)催化的交叉偶联转化,使用双齿氨基喹啉作为指向不同亲核试剂的直链基团,形成C–C,C–N和C–O键。DFT计算表明通过Ag(I)/ Ag(III)催化循环可实现L 1 -I底物的氧化加成。此外,离子光谱实验表明,在没有亲核试剂的情况下,高反应性的芳基-Ag(III)会反应形成分子间环状产物[5d-Ag(I)-CH 3 CN],其在溶液中形成5a。这项工作证明银可以在Pd和Cu等交叉偶联反应中经历2-电子氧化还原过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号