当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Harvesting the Benefits of Inherent Reactive Functionalities in Fully Biosourced Isomeric Benzoxazines
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-10-02 00:00:00 , DOI: 10.1021/acssuschemeng.8b03631 Nagarjuna Amarnath 1 , Swapnil Shukla 1 , Bimlesh Lochab 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2018-10-02 00:00:00 , DOI: 10.1021/acssuschemeng.8b03631 Nagarjuna Amarnath 1 , Swapnil Shukla 1 , Bimlesh Lochab 1
Affiliation
|
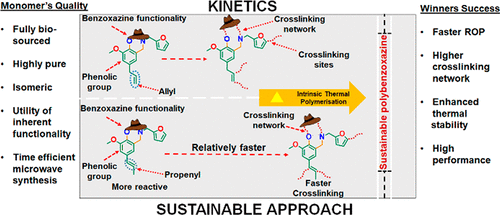
Isomerization of double bonds from an allylic to propenyl position is generally mediated by expensive metal catalysts, demanding an additional synthetic step, thereby reducing sustainability of the reaction. However, such functionalities are inherent in naturally occurring compounds, enabling a versatile protocol for their industrial utility. Herein, we report the synthesis of benzoxazine monomers based on biosourced isomeric phenols, eugenol (E) and isoeugenol (IE), and biobased amine, furfurylamine (fa) to form E-fa and IE-fa monomer, respectively. The structural variation in the phenols revealed a differential chemical reactivity, during both the synthesis of the monomer and the polymerization reaction, confirming a significant influence of isomerism. The monomers only differ in the position of the double bond in the para-substituted propylene unit forming nonconjugated vs conjugated alkylene chain with the benzene ring containing benzoxazine in E-fa and IE-fa, respectively. The structure of the monomers was confirmed by 1H NMR, 13C NMR, FTIR, XRD, and mass spectrometry. The high purity of monomer was further affirmed by HPLC and DSC to demonstrate the effect of isomerization on the polymerization behavior. The extended conjugation of the double bond in IE-fa with the proximal benzoxazine ring showed a higher reactivity toward ring-opening polymerization, polymer conversion, and cross-linking reactions as supported by FTIR, NMR, and DSC-based kinetic studies. Thermal stability, mechanical properties, and adhesive analysis by TGA, DMTA, and lap shear strength measurements further supported the effect of structural isomerism of monomers with a higher potential of PIE-fa over the PE-fa network. Current work illustrates an economic, one-step, microwave-assisted, and VOC’s- and catalyst-free synthesis with a simultaneous solventless processing of synthesized monomers using renewable materials as feedstocks for high-performance polymers.
中文翻译:

充分利用生物来源的异构异构苯并恶嗪中固有的反应性功能的收益
从烯丙基位置到丙烯基位置的双键的异构化通常是由昂贵的金属催化剂介导的,需要额外的合成步骤,从而降低了反应的可持续性。但是,此类功能是天然化合物固有的功能,可实现其工业实用性的通用协议。本文中,我们报告了基于生物来源的异构酚,丁子香酚(E)和异丁香酚(IE)以及生物胺,糠胺(fa)分别形成E-fa和IE-fa单体的苯并恶嗪单体的合成。酚的结构变化在单体的合成和聚合反应期间均显示出不同的化学反应性,证实了异构现象的显着影响。单体的区别仅在于在对位取代的丙烯单元中双键的位置不同,从而形成分别与E-fa和IE-fa中含苯并恶嗪的苯环形成非共轭或共轭的亚烷基链。单体的结构通过1 H NMR,1313 C NMR,FTIR,XRD和质谱。HPLC和DSC进一步证实了单体的高纯度,以证明异构化对聚合行为的影响。IE-fa中的双键与近端苯并恶嗪环的扩展共轭表现出更高的反应性,这受到FTIR,NMR和DSC动力学研究的支持,对开环聚合,聚合物转化和交联反应具有更高的反应性。热稳定性,机械性能以及通过TGA,DMTA和搭接剪切强度测量进行的胶粘剂分析进一步支持了在PE-fa网络上具有较高PIE-fa潜力的单体的结构异构现象的影响。当前的工作说明了一种经济的,一步一步的,微波辅助的,
更新日期:2018-10-02
中文翻译:

充分利用生物来源的异构异构苯并恶嗪中固有的反应性功能的收益
从烯丙基位置到丙烯基位置的双键的异构化通常是由昂贵的金属催化剂介导的,需要额外的合成步骤,从而降低了反应的可持续性。但是,此类功能是天然化合物固有的功能,可实现其工业实用性的通用协议。本文中,我们报告了基于生物来源的异构酚,丁子香酚(E)和异丁香酚(IE)以及生物胺,糠胺(fa)分别形成E-fa和IE-fa单体的苯并恶嗪单体的合成。酚的结构变化在单体的合成和聚合反应期间均显示出不同的化学反应性,证实了异构现象的显着影响。单体的区别仅在于在对位取代的丙烯单元中双键的位置不同,从而形成分别与E-fa和IE-fa中含苯并恶嗪的苯环形成非共轭或共轭的亚烷基链。单体的结构通过1 H NMR,1313 C NMR,FTIR,XRD和质谱。HPLC和DSC进一步证实了单体的高纯度,以证明异构化对聚合行为的影响。IE-fa中的双键与近端苯并恶嗪环的扩展共轭表现出更高的反应性,这受到FTIR,NMR和DSC动力学研究的支持,对开环聚合,聚合物转化和交联反应具有更高的反应性。热稳定性,机械性能以及通过TGA,DMTA和搭接剪切强度测量进行的胶粘剂分析进一步支持了在PE-fa网络上具有较高PIE-fa潜力的单体的结构异构现象的影响。当前的工作说明了一种经济的,一步一步的,微波辅助的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号