当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ethanol, O, and CO adsorption on Pt nanoparticles: effects of nanoparticle size and graphene support†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-10-01 00:00:00 , DOI: 10.1039/c8cp04798g L. G. Verga 1, 2, 3 , A. E. Russell 1, 2, 3 , C.-K. Skylaris 1, 2, 3
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2018-10-01 00:00:00 , DOI: 10.1039/c8cp04798g L. G. Verga 1, 2, 3 , A. E. Russell 1, 2, 3 , C.-K. Skylaris 1, 2, 3
Affiliation
|
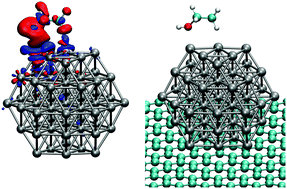
Pt nanoparticles dispersed over carbonaceous supports are widely used as catalysts for different applications, making studies on the interplay between size and support effects indispensable for rational catalyst design. Here, we use DFT calculations to simulate the interaction between O, CO, and ethanol with free platinum cuboctahedral nanoparticles with up to 147 atoms and with the same Pt nanoparticles supported on a single layer of graphene with up to 720 carbon atoms. We compute adsorption energies for each adsorbate on different adsorption sites for supported and unsupported Pt nanoparticles. We show that as the Pt nanoparticle grows the adsorption energy decreases, and that the size effect is more important for O and CO adsorption than for ethanol. We observe that the generalized coordination number of each adsorption site controls the interaction strength for O and CO to a much larger extent than for ethanol. Electronic charge redistributions and density of states projected on the d band of the interacting Pt facets are used to obtain a better understanding of the differences between the electronic interactions for each adsorbate. For Pt nanoparticles supported on graphene, the support effects weaken the adsorption energies for all the adsorbates, but this effect rapidly decreases with larger nanoparticles, and it is only significant for our smallest nanoparticle Pt13. By demonstrating that the effects of nanoparticle size and support are different for ethanol as compared with O and CO, we conclude that it should be possible to modify different parameters in the catalyst design in order to tune the Pt nanoparticle to interact with specific adsorbates.
中文翻译:

乙醇,O和CO在Pt纳米颗粒上的吸附:纳米颗粒尺寸和石墨烯载体的影响†
分散在碳质载体上的Pt纳米颗粒被广泛用作不同用途的催化剂,因此研究尺寸和载体效应之间的相互作用对于合理设计催化剂是必不可少的。在这里,我们使用DFT计算来模拟O,CO和乙醇与多达147个原子的游离铂立方八面体纳米粒子以及载于多达720个碳原子的单层石墨烯上的相同Pt纳米粒子之间的相互作用。我们计算了负载的和不负载的Pt纳米粒子在不同吸附位点上每种吸附物的吸附能。我们表明,随着Pt纳米颗粒的增长,吸附能降低,并且尺寸效应对O和CO的吸附比对乙醇的吸附更为重要。我们观察到,每个吸附位点的广义配位数控制着O和CO的相互作用强度,其程度要比乙醇大得多。电荷重分布和相互作用的Pt小平面d波段上投影的状态密度用于更好地理解每种被吸附物的电子相互作用之间的差异。对于负载在石墨烯上的Pt纳米颗粒,其支撑作用减弱了所有被吸附物的吸附能,但是这种作用随着较大的纳米颗粒而迅速降低,这仅对我们最小的纳米颗粒Pt有意义。电荷重分布和相互作用的Pt小平面d波段上投影的状态密度用于更好地理解每种被吸附物的电子相互作用之间的差异。对于负载在石墨烯上的Pt纳米颗粒,其支撑作用减弱了所有被吸附物的吸附能,但是对于较大的纳米颗粒,这种作用会迅速降低,这仅对最小的Pt纳米颗粒有意义 电荷重分布和相互作用的Pt小平面d波段上投影的状态密度用于更好地理解每种被吸附物的电子相互作用之间的差异。对于负载在石墨烯上的Pt纳米颗粒,其支撑作用减弱了所有被吸附物的吸附能,但是对于较大的纳米颗粒,这种作用会迅速降低,这仅对最小的Pt纳米颗粒有意义13。通过证明与O和CO相比,乙醇对纳米颗粒尺寸和载体的影响是不同的,我们得出结论,应该有可能在催化剂设计中修改不同的参数,以调节Pt纳米颗粒与特定的被吸附物相互作用。
更新日期:2018-10-01
中文翻译:

乙醇,O和CO在Pt纳米颗粒上的吸附:纳米颗粒尺寸和石墨烯载体的影响†
分散在碳质载体上的Pt纳米颗粒被广泛用作不同用途的催化剂,因此研究尺寸和载体效应之间的相互作用对于合理设计催化剂是必不可少的。在这里,我们使用DFT计算来模拟O,CO和乙醇与多达147个原子的游离铂立方八面体纳米粒子以及载于多达720个碳原子的单层石墨烯上的相同Pt纳米粒子之间的相互作用。我们计算了负载的和不负载的Pt纳米粒子在不同吸附位点上每种吸附物的吸附能。我们表明,随着Pt纳米颗粒的增长,吸附能降低,并且尺寸效应对O和CO的吸附比对乙醇的吸附更为重要。我们观察到,每个吸附位点的广义配位数控制着O和CO的相互作用强度,其程度要比乙醇大得多。电荷重分布和相互作用的Pt小平面d波段上投影的状态密度用于更好地理解每种被吸附物的电子相互作用之间的差异。对于负载在石墨烯上的Pt纳米颗粒,其支撑作用减弱了所有被吸附物的吸附能,但是这种作用随着较大的纳米颗粒而迅速降低,这仅对我们最小的纳米颗粒Pt有意义。电荷重分布和相互作用的Pt小平面d波段上投影的状态密度用于更好地理解每种被吸附物的电子相互作用之间的差异。对于负载在石墨烯上的Pt纳米颗粒,其支撑作用减弱了所有被吸附物的吸附能,但是对于较大的纳米颗粒,这种作用会迅速降低,这仅对最小的Pt纳米颗粒有意义 电荷重分布和相互作用的Pt小平面d波段上投影的状态密度用于更好地理解每种被吸附物的电子相互作用之间的差异。对于负载在石墨烯上的Pt纳米颗粒,其支撑作用减弱了所有被吸附物的吸附能,但是对于较大的纳米颗粒,这种作用会迅速降低,这仅对最小的Pt纳米颗粒有意义13。通过证明与O和CO相比,乙醇对纳米颗粒尺寸和载体的影响是不同的,我们得出结论,应该有可能在催化剂设计中修改不同的参数,以调节Pt纳米颗粒与特定的被吸附物相互作用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号