当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu2+ selective chelators relieve copper-induced oxidative stress in vivo†
Chemical Science ( IF 7.6 ) Pub Date : 2018-10-02 00:00:00 , DOI: 10.1039/c8sc04041a Ananya Rakshit 1 , Kaustav Khatua 1 , Vinit Shanbhag 2 , Peter Comba 3 , Ankona Datta 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-10-02 00:00:00 , DOI: 10.1039/c8sc04041a Ananya Rakshit 1 , Kaustav Khatua 1 , Vinit Shanbhag 2 , Peter Comba 3 , Ankona Datta 1
Affiliation
|
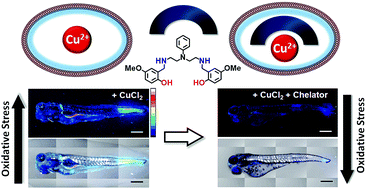
Copper ions are essential for biological function yet are severely detrimental when present in excess. At the molecular level, copper ions catalyze the production of hydroxyl radicals that can irreversibly alter essential bio-molecules. Hence, selective copper chelators that can remove excess copper ions and alleviate oxidative stress will help assuage copper-overload diseases. However, most currently available chelators are non-specific leading to multiple undesirable side-effects. The challenge is to build chelators that can bind to copper ions with high affinity but leave the levels of essential metal ions unaltered. Here we report the design and development of redox-state selective Cu ion chelators that have 108 times higher conditional stability constants toward Cu2+ compared to both Cu+ and other biologically relevant metal ions. This unique selectivity allows the specific removal of Cu2+ ions that would be available only under pathophysiological metal overload and oxidative stress conditions and provides access to effective removal of the aberrant redox-cycling Cu ion pool without affecting the essential non-redox cycling Cu+ labile pool. We have shown that the chelators provide distinct protection against copper-induced oxidative stress in vitro and in live cells via selective Cu2+ ion chelation. Notably, the chelators afford significant reduction in Cu-induced oxidative damage in Atp7a−/− Menkes disease model cells that have endogenously high levels of Cu ions. Finally, in vivo testing of our chelators in a live zebrafish larval model demonstrate their protective properties against copper-induced oxidative stress.
中文翻译:

Cu2+ 选择性螯合剂可缓解铜诱导的体内氧化应激†
铜离子对于生物功能至关重要,但过量存在会严重有害。在分子水平上,铜离子催化羟基自由基的产生,羟基自由基可以不可逆地改变重要的生物分子。因此,可以去除多余铜离子并减轻氧化应激的选择性铜螯合剂将有助于缓解铜超载疾病。然而,目前大多数可用的螯合剂都是非特异性的,导致多种不良副作用。面临的挑战是构建能够以高亲和力与铜离子结合但保持必需金属离子水平不变的螯合剂。在这里,我们报告了氧化还原态选择性铜离子螯合剂的设计和开发,该螯合剂对 Cu 2+的条件稳定性常数比 Cu +和其他生物相关金属离子高 10 8倍。这种独特的选择性允许特异性去除仅在病理生理学金属过载和氧化应激条件下才可用的 Cu 2+离子,并提供有效去除异常氧化还原循环铜离子池的途径,而不影响基本的非氧化还原循环铜离子不稳定池。我们已经证明,螯合剂通过选择性 Cu 2+离子螯合,在体外和活细胞中提供针对铜诱导的氧化应激的独特保护。值得注意的是,螯合剂可显着减少内源性高水平铜离子的 Atp7a -/−门克斯病模型细胞中铜诱导的氧化损伤。 最后,我们的螯合剂在活体斑马鱼幼虫模型中的体内测试证明了它们对铜诱导的氧化应激的保护特性。
更新日期:2018-10-02
中文翻译:

Cu2+ 选择性螯合剂可缓解铜诱导的体内氧化应激†
铜离子对于生物功能至关重要,但过量存在会严重有害。在分子水平上,铜离子催化羟基自由基的产生,羟基自由基可以不可逆地改变重要的生物分子。因此,可以去除多余铜离子并减轻氧化应激的选择性铜螯合剂将有助于缓解铜超载疾病。然而,目前大多数可用的螯合剂都是非特异性的,导致多种不良副作用。面临的挑战是构建能够以高亲和力与铜离子结合但保持必需金属离子水平不变的螯合剂。在这里,我们报告了氧化还原态选择性铜离子螯合剂的设计和开发,该螯合剂对 Cu 2+的条件稳定性常数比 Cu +和其他生物相关金属离子高 10 8倍。这种独特的选择性允许特异性去除仅在病理生理学金属过载和氧化应激条件下才可用的 Cu 2+离子,并提供有效去除异常氧化还原循环铜离子池的途径,而不影响基本的非氧化还原循环铜离子不稳定池。我们已经证明,螯合剂通过选择性 Cu 2+离子螯合,在体外和活细胞中提供针对铜诱导的氧化应激的独特保护。值得注意的是,螯合剂可显着减少内源性高水平铜离子的 Atp7a -/−门克斯病模型细胞中铜诱导的氧化损伤。 最后,我们的螯合剂在活体斑马鱼幼虫模型中的体内测试证明了它们对铜诱导的氧化应激的保护特性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号