当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mannich-type Reactions of Cyclic Nitrones: Effective Methods for the Enantioselective Synthesis of Piperidine-containing Alkaloids.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-19 , DOI: 10.1002/anie.201809799 Vladislav G Lisnyak 1 , Tessa Lynch-Colameta 1 , Scott A Snyder 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-10-19 , DOI: 10.1002/anie.201809799 Vladislav G Lisnyak 1 , Tessa Lynch-Colameta 1 , Scott A Snyder 1
Affiliation
|
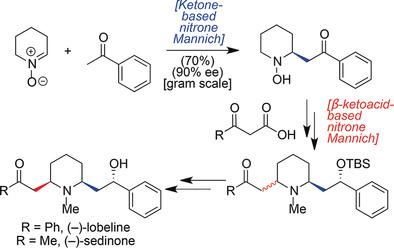
Even though there are dozens of biologically active 2-substituted and 2,6-disubstituted piperidines, only a limited number of approaches exist for their synthesis. Herein is described two Mannich-type additions to nitrones, one using β-ketoacids under catalyst-free conditions and another using methyl ketones in the presence of chiral thioureas, which can generate a broad array of such 2-substituted materials, as well as other ring variants, in the form of β-N-hydroxy-aminoketones. Both processes have broad scope, with the latter providing products with high enantioselectivity (up to 98 %). The combination of these methods, along with other critical steps, has enabled 8-step total syntheses of the 2,6-disubstituted piperidine alkaloids (-)-lobeline and (-)-sedinone.
中文翻译:

环硝酮的曼尼希型反应:对映选择性合成含哌啶生物碱的有效方法。
尽管有数十种具有生物活性的 2-取代和 2,6-二取代哌啶,但其合成方法却有限。本文描述了硝酮的两种曼尼希型加成,一种在无催化剂条件下使用β-酮酸,另一种在手性硫脲存在下使用甲基酮,这可以生成多种此类2-取代材料以及其他材料环变体,以β-N-羟基氨基酮的形式。这两种工艺都有广泛的适用范围,后者提供的产品具有高对映选择性(高达 98%)。这些方法与其他关键步骤的结合,使得 2,6-二取代哌啶生物碱 (-)-山楂碱和 (-)-sedinone 的 8 步全合成成为可能。
更新日期:2018-10-19
中文翻译:

环硝酮的曼尼希型反应:对映选择性合成含哌啶生物碱的有效方法。
尽管有数十种具有生物活性的 2-取代和 2,6-二取代哌啶,但其合成方法却有限。本文描述了硝酮的两种曼尼希型加成,一种在无催化剂条件下使用β-酮酸,另一种在手性硫脲存在下使用甲基酮,这可以生成多种此类2-取代材料以及其他材料环变体,以β-N-羟基氨基酮的形式。这两种工艺都有广泛的适用范围,后者提供的产品具有高对映选择性(高达 98%)。这些方法与其他关键步骤的结合,使得 2,6-二取代哌啶生物碱 (-)-山楂碱和 (-)-sedinone 的 8 步全合成成为可能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号