Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-09-30 , DOI: 10.1016/j.jfluchem.2018.09.007 Minhui Yu , Yueci Wu , Xin Peng , Jing Han , Jie Chen , Yuhe Kan , Hongmei Deng , Min Shao , Hui Zhang , Weiguo Cao
|
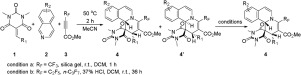
The 1,4-dipoles derived from isoquinolines and methyl perfluoroalk-2-ynoates reacted readily with arylidene-substituted N,N-dimethylbarbituric acids resulting in the first diastereoselective synthesis of perfluoroalkylated cis-spiropyrido[2,1-a]isoquinoline-1,5’-pyrimidine derivatives in good to excellent yields under mild conditions. The reaction mechanism was proposed to illustrate the formation of the diastereoisomers and proton-promoted transformation of trans-spiropyrido[2,1-a]isoquinoline-1,5’-pyrimidines to the more thermodynamically stable cis-isomers. The DFT calculation demonstrated the diastereoselectivity of the reaction.
中文翻译:

全氟烷基化的顺式-吡咯并吡啶并[ 2,1- a ]异喹啉-1,5'-嘧啶的非对映选择性合成
衍生自异喹啉和全氟烷基-2-炔酸甲酯的1,4-偶极易于与亚芳基取代的N,N-二甲基巴比妥酸反应,导致全氟烷基化的顺式-吡咯并[2,1- a ]异喹啉-1的第一个非对映选择性合成, 5'-嘧啶衍生物在温和条件下的产率高至优异。提出了反应机理以说明非对映异构体的形成和反式-吡咯并[ 2,1- a ]异喹啉-1,5'-嘧啶质子促进的转化为热力学更稳定的顺式异构体。DFT计算证明了反应的非对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号