当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergy between Defects, Photoexcited Electrons, and Supported Single Atom Catalysts for CO2 Reduction
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-01 00:00:00 , DOI: 10.1021/acscatal.8b02372 Junbo Chen 1 , Satish Kumar Iyemperumal 1 , Thomas Fenton 2 , Alexander Carl 3 , Ronald Grimm 3 , Gonghu Li 2 , N. Aaron Deskins 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-01 00:00:00 , DOI: 10.1021/acscatal.8b02372 Junbo Chen 1 , Satish Kumar Iyemperumal 1 , Thomas Fenton 2 , Alexander Carl 3 , Ronald Grimm 3 , Gonghu Li 2 , N. Aaron Deskins 1
Affiliation
|
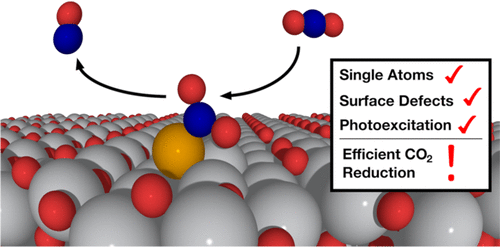
Dispersed atomic catalysts can achieve high catalytic efficiency and have the potential to enable chemical transformation of inert molecules like CO2. The effect of surface defects on photocatalytic reduction of CO2 using supported single atom catalysts however requires clarification. Using density functional theory and experimental techniques, we have investigated the role of surface oxygen vacancies (Ov) and photoexcited electrons on supported single atom Cu catalysts and CO2 reduction. Adsorption of Cu was strong to the TiO2 surface, and charges of the Cu atoms were highly dependent on whether surface defects were present. Cu atoms with Ov aided in the adsorption of activated bent CO2, which is key to CO2 reduction. Our results also show that CO2 dissociation (CO2* → CO* + O*), which is a proposed initial step of CO2 reduction to hydrocarbon products, occurs very readily for a single Cu atom in an Ov, with barriers of ∼0.19 eV. Such low barriers do not occur with Cu over a stoichiometric surface. Furthermore, the presence of a photoexcited electron leads to a substantial increase in reaction rate for Cu over a stoichiometric surface; the Cu/TiO2 surface is largely inert in the absence of photoexcited electrons. Experimental results corroborate these theoretical calculations and show that activation of CO2 occurs most readily for TiO2 catalysts with dispersed Cu and Ov. CO2 photoreduction also occurs most readily for TiO2 catalysts with dispersed Cu and Ov, compared to TiO2 or Cu over stoichiometric TiO2 catalysts. We also modeled atomic Pt to understand how metals besides Cu may behave. We found that Pt over TiO2 also activates CO2 but that dissociation of CO2 over Pt with Ov does not occur as readily as for Cu with Ov. Our results show that tailoring TiO2 surfaces with defects in conjunction with specific atomic catalysts like Cu may lead to fast desirable photoreduction of CO2.
中文翻译:

缺陷,光激发电子和负载型单原子催化剂之间用于CO 2还原的协同作用
分散的原子催化剂可以实现高催化效率,并且具有实现惰性分子(如CO 2)化学转化的潜力。然而,需要澄清表面缺陷对使用负载型单原子催化剂的CO 2光催化还原的影响。使用密度泛函理论和实验技术,我们研究了表面氧空位(O v)和光激发电子在负载型单原子Cu催化剂和CO 2还原上的作用。Cu对TiO 2表面的吸附很强,并且Cu原子的电荷高度依赖于是否存在表面缺陷。带有O v的Cu原子有助于吸附活性弯曲的CO 2,这是减少CO 2的关键。我们的结果还表明,CO 2的解离(CO 2 *→CO * + O *)是将CO 2还原为烃类产物的拟议初始步骤,对于O v中的单个Cu原子,很容易发生隔离,约0.19 eV。在化学计量表面上,Cu不会发生这种低势垒。此外,光激发电子的存在导致化学计量表面上Cu的反应速率大大提高;铜/钛2在没有光激发电子的情况下,表面很大程度上是惰性的。实验结果证实了这些理论计算结果,结果表明,分散有Cu和O v的TiO 2催化剂最容易发生CO 2活化。与化学计量的TiO 2催化剂上的TiO 2或Cu相比,具有分散的Cu和O v的TiO 2催化剂也最容易发生CO 2光还原。我们还对原子Pt建模,以了解除Cu以外的金属的行为方式。我们发现,铂在二氧化钛2也激活CO 2,但CO的那离2在Pt为O v不像O v的Cu那样容易发生。我们的结果表明,与特定的原子催化剂(如Cu)一起对具有缺陷的TiO 2表面进行修整可能会导致快速理想的CO 2光还原。
更新日期:2018-10-01
中文翻译:

缺陷,光激发电子和负载型单原子催化剂之间用于CO 2还原的协同作用
分散的原子催化剂可以实现高催化效率,并且具有实现惰性分子(如CO 2)化学转化的潜力。然而,需要澄清表面缺陷对使用负载型单原子催化剂的CO 2光催化还原的影响。使用密度泛函理论和实验技术,我们研究了表面氧空位(O v)和光激发电子在负载型单原子Cu催化剂和CO 2还原上的作用。Cu对TiO 2表面的吸附很强,并且Cu原子的电荷高度依赖于是否存在表面缺陷。带有O v的Cu原子有助于吸附活性弯曲的CO 2,这是减少CO 2的关键。我们的结果还表明,CO 2的解离(CO 2 *→CO * + O *)是将CO 2还原为烃类产物的拟议初始步骤,对于O v中的单个Cu原子,很容易发生隔离,约0.19 eV。在化学计量表面上,Cu不会发生这种低势垒。此外,光激发电子的存在导致化学计量表面上Cu的反应速率大大提高;铜/钛2在没有光激发电子的情况下,表面很大程度上是惰性的。实验结果证实了这些理论计算结果,结果表明,分散有Cu和O v的TiO 2催化剂最容易发生CO 2活化。与化学计量的TiO 2催化剂上的TiO 2或Cu相比,具有分散的Cu和O v的TiO 2催化剂也最容易发生CO 2光还原。我们还对原子Pt建模,以了解除Cu以外的金属的行为方式。我们发现,铂在二氧化钛2也激活CO 2,但CO的那离2在Pt为O v不像O v的Cu那样容易发生。我们的结果表明,与特定的原子催化剂(如Cu)一起对具有缺陷的TiO 2表面进行修整可能会导致快速理想的CO 2光还原。











































 京公网安备 11010802027423号
京公网安备 11010802027423号