当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electron and Hydride Transfer in a Redox-Active NiFe Hydride Complex: A DFT Study
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-09-28 00:00:00 , DOI: 10.1021/acscatal.8b02368 Miho Isegawa 1, 2 , Akhilesh K. Sharma 2 , Seiji Ogo 1 , Keiji Morokuma 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-09-28 00:00:00 , DOI: 10.1021/acscatal.8b02368 Miho Isegawa 1, 2 , Akhilesh K. Sharma 2 , Seiji Ogo 1 , Keiji Morokuma 2
Affiliation
|
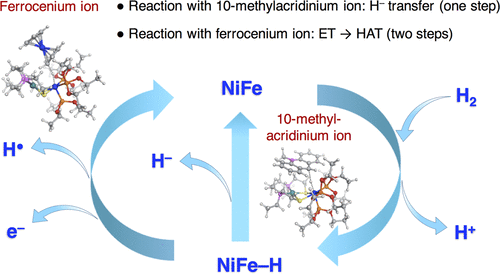
We present mechanistic details of the formation of a NiFe hydride complex and provide information on its electron- and hydride-transfer processes on the basis of density functional theory calculations and artificial-force-induced-reaction studies. The NiFe hydride complex conducts three transfer reactions: namely, electron transfer, hydride transfer, and proton transfer. In a NiFe hydride complex, the hydride binds to Fe, which is different from the Ni–R state in hydrogenase where the hydride is located between Ni and Fe. According to our calculations, in reaction with the ferrocenium ion, electron transfer occurs from the NiFe hydride complex to the ferrocenium ion, followed by a hydrogen atom transfer (HAT) to the second ferrocenium ion. The oxidation state of Fe varies during the redox process, different from the case of NiFe hydrogenase, where the oxidation state of Ni varies. A single-step hydride transfer occurs in the presence of a 10-methylacridinium ion (AcrH+), which is more kinetically feasible than the HAT process. In contrast to the HAT and hydride-transfer process, the proton transfer occurs through a low barrier from a protonated diethyl ether. The revealed reaction mechanism guides the interpretation of the catalytic cycle of NiFe hydrogenase and leads to the development of efficient biomimetic catalysts for H2 generation and an electron/hydride transfer.
中文翻译:

氧化还原活性镍铁氢化物络合物中的电子和氢化物转移:DFT研究
我们介绍了镍铁氢化物配合物形成的机理细节,并基于密度泛函理论计算和人工诱导反应研究提供了有关其电子和氢化物转移过程的信息。氢化镍铁配合物进行三个转移反应:电子转移,氢化物转移和质子转移。在NiFe氢化物络合物中,氢化物与Fe结合,这不同于氢化物位于Ni和Fe之间的氢化酶中的Ni–R状态。根据我们的计算,在与二茂铁离子反应时,发生了电子从NiFe氢化物络合物到二茂铁离子的转移,然后是氢原子转移(HAT)到第二茂铁离子的转移。Fe的氧化态在氧化还原过程中会发生变化,这与NiFe氢化酶的情况不同,Ni的氧化态变化。在10-甲基ac离子(AcrH+),在动力学上比HAT过程更可行。与HAT和氢化物转移过程相反,质子转移通过来自质子化乙醚的低势垒发生。揭示的反应机理指导了NiFe氢化酶催化循环的解释,并导致了用于H 2生成和电子/氢化物转移的高效仿生催化剂的发展。
更新日期:2018-09-28
中文翻译:

氧化还原活性镍铁氢化物络合物中的电子和氢化物转移:DFT研究
我们介绍了镍铁氢化物配合物形成的机理细节,并基于密度泛函理论计算和人工诱导反应研究提供了有关其电子和氢化物转移过程的信息。氢化镍铁配合物进行三个转移反应:电子转移,氢化物转移和质子转移。在NiFe氢化物络合物中,氢化物与Fe结合,这不同于氢化物位于Ni和Fe之间的氢化酶中的Ni–R状态。根据我们的计算,在与二茂铁离子反应时,发生了电子从NiFe氢化物络合物到二茂铁离子的转移,然后是氢原子转移(HAT)到第二茂铁离子的转移。Fe的氧化态在氧化还原过程中会发生变化,这与NiFe氢化酶的情况不同,Ni的氧化态变化。在10-甲基ac离子(AcrH+),在动力学上比HAT过程更可行。与HAT和氢化物转移过程相反,质子转移通过来自质子化乙醚的低势垒发生。揭示的反应机理指导了NiFe氢化酶催化循环的解释,并导致了用于H 2生成和电子/氢化物转移的高效仿生催化剂的发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号