Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Erythrocyte Membrane Cloaked Metal–Organic Framework Nanoparticle as Biomimetic Nanoreactor for Starvation-Activated Colon Cancer Therapy
ACS Nano ( IF 15.8 ) Pub Date : 2018-09-28 00:00:00 , DOI: 10.1021/acsnano.8b05200 Lu Zhang 1, 2 , Zhenzhen Wang 1, 2 , Yan Zhang 1, 2 , Fangfang Cao 1, 2 , Kai Dong 1 , Jinsong Ren 1 , Xiaogang Qu 1
ACS Nano ( IF 15.8 ) Pub Date : 2018-09-28 00:00:00 , DOI: 10.1021/acsnano.8b05200 Lu Zhang 1, 2 , Zhenzhen Wang 1, 2 , Yan Zhang 1, 2 , Fangfang Cao 1, 2 , Kai Dong 1 , Jinsong Ren 1 , Xiaogang Qu 1
Affiliation
|
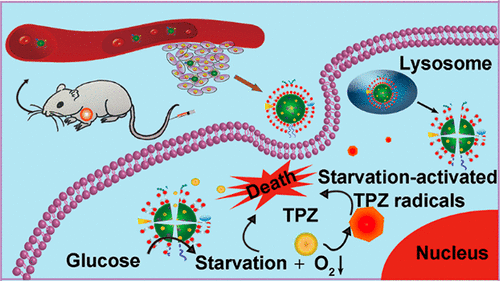
Shutting down glucose supply by glucose oxidase (GOx) to starve tumors has been considered to be an attractive strategy in cancerous starvation therapy. Nevertheless, the in vivo applications of GOx-based starvation therapy are severely restricted by the poor GOx delivery efficiency and the self-limiting therapeutic effect. Herein, a biomimetic nanoreactor has been fabricated for starvation-activated cancer therapy by encapsulating GOx and prodrug tirapazamine (TPZ) in an erythrocyte membrane cloaked metal–organic framework (MOF) nanoparticle ([email protected]). The fabricated [email protected] nanoreactor can assist the delivery of GOx to tumor cells and then exhaust endogenous glucose and O2 to starve tumors efficiently. Importantly, the resulting tumor hypoxia by GOx-based starvation therapy further initiates the activation of TPZ, which is released from the nanoreactor in the acid lyso/endosome environment, for enhanced colon cancer therapy. More importantly, by integrating the biomimetic surface modification, the immunity-escaping and prolonged blood circulation characteristics endow our nanoreactor dramatically improved cancer targeting ability. The in vitro and in vivo outcomes indicate our biomimetic nanoreactor exhibits a strong synergistic cascade effect for colon cancer therapy in an accurate and facile manner.
中文翻译:

红细胞膜隐身的金属有机框架纳米粒子作为仿生纳米反应器用于饥饿激活的结肠癌治疗。
通过葡萄糖氧化酶(GOx)切断葡萄糖供应以使肿瘤饥饿已被认为是癌性饥饿疗法中的一种有吸引力的策略。然而,基于GOx的饥饿疗法的体内应用受到差的GOx递送效率和自我限制的治疗效果的严重限制。本文中,通过将GOx和前药替拉帕明(TPZ)封装在红细胞膜掩盖的金属有机框架(MOF)纳米颗粒中,制造了一种仿生纳米反应器,用于饥饿激活的癌症治疗([电子邮件保护])。制成的[受电子邮件保护的]纳米反应器可以协助将GOx输送至肿瘤细胞,然后排出内源性葡萄糖和O 2有效地使肿瘤饿死。重要的是,基于GOx的饥饿疗法导致的肿瘤缺氧进一步引发了TPZ的活化,TPZ的活化是从酸性溶酶/内体环境中的纳米反应器释放出来的,用于增强结肠癌的治疗。更重要的是,通过整合仿生表面修饰,提高了免疫力和延长了血液循环的特性,使我们的纳米反应器大大提高了癌症靶向能力。在体外和体内结果表明我们的仿生纳米反应器显示出对结肠癌治疗中的准确和容易的方式强烈的协同级联效应。
更新日期:2018-09-28
中文翻译:

红细胞膜隐身的金属有机框架纳米粒子作为仿生纳米反应器用于饥饿激活的结肠癌治疗。
通过葡萄糖氧化酶(GOx)切断葡萄糖供应以使肿瘤饥饿已被认为是癌性饥饿疗法中的一种有吸引力的策略。然而,基于GOx的饥饿疗法的体内应用受到差的GOx递送效率和自我限制的治疗效果的严重限制。本文中,通过将GOx和前药替拉帕明(TPZ)封装在红细胞膜掩盖的金属有机框架(MOF)纳米颗粒中,制造了一种仿生纳米反应器,用于饥饿激活的癌症治疗([电子邮件保护])。制成的[受电子邮件保护的]纳米反应器可以协助将GOx输送至肿瘤细胞,然后排出内源性葡萄糖和O 2有效地使肿瘤饿死。重要的是,基于GOx的饥饿疗法导致的肿瘤缺氧进一步引发了TPZ的活化,TPZ的活化是从酸性溶酶/内体环境中的纳米反应器释放出来的,用于增强结肠癌的治疗。更重要的是,通过整合仿生表面修饰,提高了免疫力和延长了血液循环的特性,使我们的纳米反应器大大提高了癌症靶向能力。在体外和体内结果表明我们的仿生纳米反应器显示出对结肠癌治疗中的准确和容易的方式强烈的协同级联效应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号