当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Dominant Role of Entropy in Stabilizing Sugar Isomerization Transition States within Hydrophobic Zeolite Pores
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-09-28 , DOI: 10.1021/jacs.8b08336 Michael J. Cordon 1 , James W. Harris 1 , Juan Carlos Vega-Vila 1 , Jason S. Bates 1 , Sukhdeep Kaur 2 , Mohit Gupta 2 , Megan E. Witzke 3 , Evan C. Wegener 1 , Jeffrey T. Miller 1 , David W. Flaherty 3 , David D. Hibbitts 2 , Rajamani Gounder 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-09-28 , DOI: 10.1021/jacs.8b08336 Michael J. Cordon 1 , James W. Harris 1 , Juan Carlos Vega-Vila 1 , Jason S. Bates 1 , Sukhdeep Kaur 2 , Mohit Gupta 2 , Megan E. Witzke 3 , Evan C. Wegener 1 , Jeffrey T. Miller 1 , David W. Flaherty 3 , David D. Hibbitts 2 , Rajamani Gounder 1
Affiliation
|
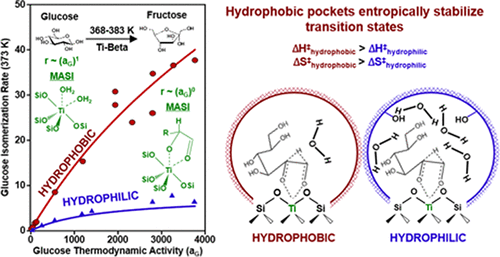
Lewis acid sites in zeolites catalyze aqueous-phase sugar isomerization at higher turnover rates when confined within hydrophobic rather than within hydrophilic micropores; however, relative contributions of competitive water adsorption at active sites and preferential stabilization of isomerization transition states have remained unclear. Here, we employ a suite of experimental and theoretical techniques to elucidate the effects of coadsorbed water on glucose isomerization reaction coordinate free energy landscapes. Transmission IR spectra provide evidence that water forms extended hydrogen-bonding networks within hydrophilic but not hydrophobic micropores of Beta zeolites. Aqueous-phase glucose isomerization turnover rates measured on Ti-Beta zeolites transition from first-order to zero-order dependence on glucose thermodynamic activity, as Lewis acidic Ti sites transition from water-covered to glucose-covered, consistent with intermediates identified from modulation excitation spectroscopy during in situ attenuated total reflectance IR experiments. First-order and zero-order isomerization rate constants are systematically higher (by 3-12×, 368-383 K) when Ti sites are confined within hydrophobic micropores. Apparent activation enthalpies and entropies reveal that glucose and water competitive adsorption at Ti sites depend weakly on confining environment polarity, while Gibbs free energies of hydride-shift isomerization transition states are lower when confined within hydrophobic micropores. DFT calculations suggest that interactions between intraporous water and isomerization transition states increase effective transition state sizes through second-shell solvation spheres, reducing primary solvation sphere flexibility. These findings clarify the effects of hydrophobic pockets on the stability of coadsorbed water and isomerization transition states and suggest design strategies that modify micropore polarity to influence turnover rates in liquid water.
中文翻译:

熵在稳定疏水沸石孔内糖异构化转变状态中的主要作用
沸石中的路易斯酸位点被限制在疏水性微孔内而不是亲水性微孔内时,以更高的转化率催化水相糖异构化;然而,活性位点的竞争性吸水和异构化过渡态的优先稳定的相对贡献仍不清楚。在这里,我们采用了一套实验和理论技术来阐明共吸附水对葡萄糖异构化反应坐标自由能景观的影响。透射红外光谱提供证据表明,水在 Beta 沸石的亲水性而非疏水性微孔内形成扩展的氢键网络。在 Ti-Beta 沸石上测量的水相葡萄糖异构化转换率从一级到零级的转变依赖于葡萄糖热力学活性,因为路易斯酸性 Ti 位点从水覆盖到葡萄糖覆盖,这与原位衰减全反射红外实验期间从调制激发光谱中确定的中间体一致。当 Ti 位点被限制在疏水微孔内时,一级和零级异构化速率常数系统性地更高(3-12×,368-383 K)。表观活化焓和熵表明,Ti 位点处的葡萄糖和水竞争吸附对限制环境极性的依赖性较弱,而当限制在疏水微孔内时,氢化物位移异构化过渡态的吉布斯自由能较低。DFT 计算表明,内孔水和异构化过渡态之间的相互作用通过第二壳溶剂化球增加了有效的过渡态大小,降低初级溶剂化球的灵活性。这些发现阐明了疏水口袋对共吸附水稳定性和异构化过渡态的影响,并提出了修改微孔极性以影响液态水中周转率的设计策略。
更新日期:2018-09-28
中文翻译:

熵在稳定疏水沸石孔内糖异构化转变状态中的主要作用
沸石中的路易斯酸位点被限制在疏水性微孔内而不是亲水性微孔内时,以更高的转化率催化水相糖异构化;然而,活性位点的竞争性吸水和异构化过渡态的优先稳定的相对贡献仍不清楚。在这里,我们采用了一套实验和理论技术来阐明共吸附水对葡萄糖异构化反应坐标自由能景观的影响。透射红外光谱提供证据表明,水在 Beta 沸石的亲水性而非疏水性微孔内形成扩展的氢键网络。在 Ti-Beta 沸石上测量的水相葡萄糖异构化转换率从一级到零级的转变依赖于葡萄糖热力学活性,因为路易斯酸性 Ti 位点从水覆盖到葡萄糖覆盖,这与原位衰减全反射红外实验期间从调制激发光谱中确定的中间体一致。当 Ti 位点被限制在疏水微孔内时,一级和零级异构化速率常数系统性地更高(3-12×,368-383 K)。表观活化焓和熵表明,Ti 位点处的葡萄糖和水竞争吸附对限制环境极性的依赖性较弱,而当限制在疏水微孔内时,氢化物位移异构化过渡态的吉布斯自由能较低。DFT 计算表明,内孔水和异构化过渡态之间的相互作用通过第二壳溶剂化球增加了有效的过渡态大小,降低初级溶剂化球的灵活性。这些发现阐明了疏水口袋对共吸附水稳定性和异构化过渡态的影响,并提出了修改微孔极性以影响液态水中周转率的设计策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号