当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereo- and Enantioselective Synthesis of Fluorine Motifs with Two Contiguous Stereogenic Centers
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-09-28 , DOI: 10.1021/jacs.8b08778 Sudipta Ponra 1 , Wangchuk Rabten 1 , Jianping Yang 1 , Haibo Wu 1 , Sutthichat Kerdphon 1 , Pher G. Andersson 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-09-28 , DOI: 10.1021/jacs.8b08778 Sudipta Ponra 1 , Wangchuk Rabten 1 , Jianping Yang 1 , Haibo Wu 1 , Sutthichat Kerdphon 1 , Pher G. Andersson 1
Affiliation
|
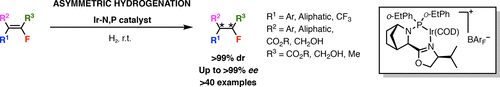
The synthesis of chiral fluorine containing motifs, in particular, chiral fluorine molecules with two contiguous stereogenic centers, has attracted much interest in research due to the limited number of methods available for their preparation. Herein, we report an atom-economical and highly stereoselective synthesis of chiral fluorine molecules with two contiguous stereogenic centers via azabicyclo iridium-oxazoline-phosphine-catalyzed hydrogenation of readily available vinyl fluorides. Various aromatic, aliphatic, and heterocyclic systems with a variety of functional groups were found to be compatible with the reaction and provide the highly desirable product as single diastereomers with excellent enantioselectivities.
中文翻译:

具有两个连续立体中心的氟基团的非对映选择性和对映选择性合成
手性氟的合成,特别是具有两个连续立体中心的手性氟分子,由于其制备方法的数量有限,引起了人们对研究的极大兴趣。在此,我们报告了通过氮杂双环铱-恶唑啉-膦催化氢化容易获得的氟乙烯,以原子经济和高度立体选择性合成具有两个连续立体中心的手性氟分子。发现具有各种官能团的各种芳香族、脂肪族和杂环系统与反应相容,并提供非常理想的产物,作为具有优异对映选择性的单一非对映异构体。
更新日期:2018-09-28
中文翻译:

具有两个连续立体中心的氟基团的非对映选择性和对映选择性合成
手性氟的合成,特别是具有两个连续立体中心的手性氟分子,由于其制备方法的数量有限,引起了人们对研究的极大兴趣。在此,我们报告了通过氮杂双环铱-恶唑啉-膦催化氢化容易获得的氟乙烯,以原子经济和高度立体选择性合成具有两个连续立体中心的手性氟分子。发现具有各种官能团的各种芳香族、脂肪族和杂环系统与反应相容,并提供非常理想的产物,作为具有优异对映选择性的单一非对映异构体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号