当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Glycoproteomic Alterations in Drug-Resistant Nonsmall Cell Lung Cancer Cells Revealed by Lectin Magnetic Nanoprobe-Based Mass Spectrometry
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2018-10-17 , DOI: 10.1021/acs.jproteome.8b00433 Juanilita T. Waniwan,Yi-Ju Chen,Rey Capangpangan,Shao-Hsing Weng,Yu-Ju Chen
Journal of Proteome Research ( IF 4.4 ) Pub Date : 2018-10-17 , DOI: 10.1021/acs.jproteome.8b00433 Juanilita T. Waniwan,Yi-Ju Chen,Rey Capangpangan,Shao-Hsing Weng,Yu-Ju Chen
|
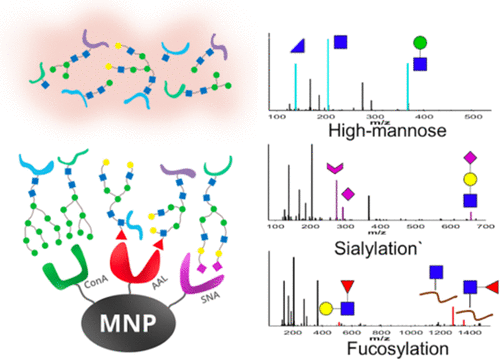
Understanding the functional role of glycosylation-mediated pathogenesis requires deep characterization of glycoproteome, which remains extremely challenging due to the inherently complex nature of glycoproteins. We demonstrate the utility of lectin–magnetic nanoprobe (MNP@lectin) coupled to Orbitrap HCD-CID-MS/MS for complementary glycotope-specific enrichment and site-specific glycosylation analysis of the glycoproteome. By three nanoprobes, MNP@ConA, MNP@AAL, and MNP@SNA, our results revealed the first large-scale glycoproteome of nonsmall cell lung cancer (NSCLC) with 2290 and 2767 nonredundant glycopeptides confidently identified (Byonic score ≥100) in EGFR-TKI-sensitive PC9 and -resistant PC9-IR cells, respectively, especially with more fucosylated and sialylated glycopeptides in PC9-IR cells. The complementary enrichment was demonstrated with only five glycopeptides commonly enriched in three MNP@lectins. Glycotope specificity of 79 and 62% for enrichment was achieved using MNP@AAL and MNP@SNA, respectively. Label-free quantitation revealed predominant fucosylation in PC9-IR cells, suggesting its potential role associated with NSCLC resistance. Moreover, without immunoprecipitation, this multilectin nanoprobe allows the sensitive identification of 51 glycopeptides from 10 of 12 reported sites from onco-protein EGFR. Our results not only demonstrated a sensitive approach to study the vastly under-represented N-glycoprotome but also may pave the way for a glycoproteomic atlas to further explore the site-specific function of glycoproteins associated with drug resistance in NSCLC.
中文翻译:

凝集素基于磁性纳米探针的质谱法揭示抗药性非小细胞肺癌细胞的糖皮质激素变化。
理解糖基化介导的发病机制的功能作用需要对糖蛋白组进行深入的表征,由于糖蛋白固有的复杂性,其仍极具挑战性。我们展示了结合Orbitrap HCD-CID-MS / MS的凝集素-磁性纳米探针(MNP @ lectin)的效用,可用于糖蛋白组的互补糖位特异性富集和位点特异性糖基化分析。通过三个纳米探针,MNP @ ConA,MNP @ AAL和MNP @ SNA,我们的结果揭示了第一个大规模的非小细胞肺癌(NSCLC)糖蛋白组,其在EGFR中确信地鉴定了2290和2767个非冗余糖肽(Byonic评分≥100)。 -TKI敏感的PC9和耐药的PC9-IR细胞,分别在PC9-IR细胞中具有更多的岩藻糖基化和唾液酸化糖肽。仅用通常在三个MNP @ lectins中富集的五个糖肽就证明了互补富集。使用MNP @ AAL和MNP @ SNA分别实现了79和62%的糖基化富集特异性。无标记定量显示PC9-IR细胞中岩藻糖基化占主导地位,表明其与NSCLC耐药性有关的潜在作用。此外,在没有免疫沉淀的情况下,这种多凝集素纳米探针可以从癌蛋白EGFR的12个报道位点中的10个中敏感鉴定51种糖肽。我们的结果不仅表明了一种敏感的方法来研究未充分代表的人群 无标记定量显示PC9-IR细胞中岩藻糖基化占主导地位,表明其与NSCLC耐药性有关的潜在作用。此外,在没有免疫沉淀的情况下,这种多凝集素纳米探针可以从癌蛋白EGFR的12个报道位点中的10个中敏感鉴定51种糖肽。我们的结果不仅表明了一种敏感的方法来研究未充分代表的人群 无标记定量显示PC9-IR细胞中岩藻糖基化占主导地位,表明其与NSCLC耐药性有关的潜在作用。此外,在没有免疫沉淀的情况下,这种多凝集素纳米探针可以从癌蛋白EGFR的12个报道位点中的10个中敏感鉴定51种糖肽。我们的结果不仅表明了一种敏感的方法来研究未充分代表的人群N-糖原蛋白也可以为糖蛋白组图谱进一步探索与NSCLC耐药相关的糖蛋白的位点特异性功能铺平道路。
更新日期:2018-10-18
中文翻译:

凝集素基于磁性纳米探针的质谱法揭示抗药性非小细胞肺癌细胞的糖皮质激素变化。
理解糖基化介导的发病机制的功能作用需要对糖蛋白组进行深入的表征,由于糖蛋白固有的复杂性,其仍极具挑战性。我们展示了结合Orbitrap HCD-CID-MS / MS的凝集素-磁性纳米探针(MNP @ lectin)的效用,可用于糖蛋白组的互补糖位特异性富集和位点特异性糖基化分析。通过三个纳米探针,MNP @ ConA,MNP @ AAL和MNP @ SNA,我们的结果揭示了第一个大规模的非小细胞肺癌(NSCLC)糖蛋白组,其在EGFR中确信地鉴定了2290和2767个非冗余糖肽(Byonic评分≥100)。 -TKI敏感的PC9和耐药的PC9-IR细胞,分别在PC9-IR细胞中具有更多的岩藻糖基化和唾液酸化糖肽。仅用通常在三个MNP @ lectins中富集的五个糖肽就证明了互补富集。使用MNP @ AAL和MNP @ SNA分别实现了79和62%的糖基化富集特异性。无标记定量显示PC9-IR细胞中岩藻糖基化占主导地位,表明其与NSCLC耐药性有关的潜在作用。此外,在没有免疫沉淀的情况下,这种多凝集素纳米探针可以从癌蛋白EGFR的12个报道位点中的10个中敏感鉴定51种糖肽。我们的结果不仅表明了一种敏感的方法来研究未充分代表的人群 无标记定量显示PC9-IR细胞中岩藻糖基化占主导地位,表明其与NSCLC耐药性有关的潜在作用。此外,在没有免疫沉淀的情况下,这种多凝集素纳米探针可以从癌蛋白EGFR的12个报道位点中的10个中敏感鉴定51种糖肽。我们的结果不仅表明了一种敏感的方法来研究未充分代表的人群 无标记定量显示PC9-IR细胞中岩藻糖基化占主导地位,表明其与NSCLC耐药性有关的潜在作用。此外,在没有免疫沉淀的情况下,这种多凝集素纳米探针可以从癌蛋白EGFR的12个报道位点中的10个中敏感鉴定51种糖肽。我们的结果不仅表明了一种敏感的方法来研究未充分代表的人群N-糖原蛋白也可以为糖蛋白组图谱进一步探索与NSCLC耐药相关的糖蛋白的位点特异性功能铺平道路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号