当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis for Highly Efficient Production of Catechol Derivatives at Acidic pH by Tyrosinase from Burkholderia thailandensis
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1021/acscatal.8b02635 Hyeoncheol Francis Son , Sang-Hyuk Lee , Seul Hoo Lee , Hyun Kim , Hwaseok Hong , Uk-Jae Lee , Pyung-Gang Lee , Byung-Gee Kim , Kyung-Jin Kim
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1021/acscatal.8b02635 Hyeoncheol Francis Son , Sang-Hyuk Lee , Seul Hoo Lee , Hyun Kim , Hwaseok Hong , Uk-Jae Lee , Pyung-Gang Lee , Byung-Gee Kim , Kyung-Jin Kim
|
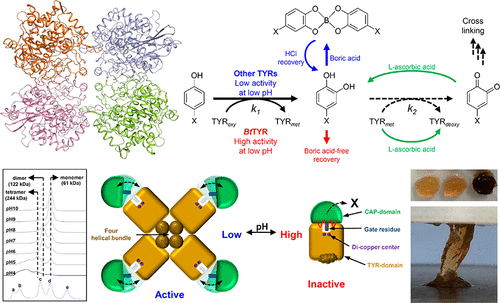
Tyrosinases (TYRs) catalyze two-step consecutive oxidation reactions of monophenolic compounds. Since known TYRs have optimal pH at neutral to somewhat basic pH, they have limitations to be used for production of catechol derivatives. In this study, we identified TYR from Burkholderia thailandensis (BtTYR), which exhibited high tyrosinase activity at low pH. We determined the crystal structure of BtTYR and provided the structural basis for the regulation of its activity in response to pH change. At high pH, BtTYR is inactivated due to the tight binding of its TYR and CAP domains, although it is stable in monomer form; at low pH, however, the protein is activated by the typical opening of the CAP domain, and the formation of tetramers maintains the stability of the protein. Such unique tyrosinase activity of BtTYR at acidic pH was successfully applied to highly efficient production of catechol derivatives and fabrication of an adhesive hydrogel.
中文翻译:

泰国伯克霍尔德菌中酪氨酸酶在酸性条件下高效生产邻苯二酚衍生物的结构基础
酪氨酸酶(TYRs)催化单酚化合物的两步连续氧化反应。由于已知的TYR在中性至基本碱性的pH值下具有最佳pH值,因此它们在生产邻苯二酚衍生物方面存在局限性。在这项研究中,我们鉴定了来自泰国伯克霍尔德菌(Bt TYR)的TYR,该菌在低pH下表现出高酪氨酸酶活性。我们确定了Bt TYR的晶体结构,并为响应pH变化而调节其活性提供了结构基础。在高pH下,BtTYR由于其TYR和CAP域紧密结合而失活,尽管它以单体形式稳定。然而,在低pH下,该蛋白质会通过CAP结构域的典型开放而被激活,四聚体的形成可维持该蛋白质的稳定性。Bt TYR在酸性pH下的这种独特的酪氨酸酶活性已成功地用于高效生产邻苯二酚衍生物和制备粘性水凝胶。
更新日期:2018-09-27
中文翻译:

泰国伯克霍尔德菌中酪氨酸酶在酸性条件下高效生产邻苯二酚衍生物的结构基础
酪氨酸酶(TYRs)催化单酚化合物的两步连续氧化反应。由于已知的TYR在中性至基本碱性的pH值下具有最佳pH值,因此它们在生产邻苯二酚衍生物方面存在局限性。在这项研究中,我们鉴定了来自泰国伯克霍尔德菌(Bt TYR)的TYR,该菌在低pH下表现出高酪氨酸酶活性。我们确定了Bt TYR的晶体结构,并为响应pH变化而调节其活性提供了结构基础。在高pH下,BtTYR由于其TYR和CAP域紧密结合而失活,尽管它以单体形式稳定。然而,在低pH下,该蛋白质会通过CAP结构域的典型开放而被激活,四聚体的形成可维持该蛋白质的稳定性。Bt TYR在酸性pH下的这种独特的酪氨酸酶活性已成功地用于高效生产邻苯二酚衍生物和制备粘性水凝胶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号