当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polyethylenimine–Spherical Nucleic Acid Nanoparticles against Gli1 Reduce the Chemoresistance and Stemness of Glioblastoma Cells
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00707 Jilian R Melamed , Stephen A Ioele , Ariel J Hannum , Violet M Ullman , Emily S Day 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-09-27 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00707 Jilian R Melamed , Stephen A Ioele , Ariel J Hannum , Violet M Ullman , Emily S Day 1
Affiliation
|
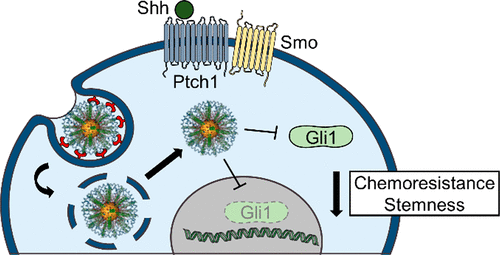
Glioblastoma (GBM) is the most common and lethal primary brain tumor in adults, with nearly 100% of patients ultimately succumbing to the disease. Median patient survival is 15 months, and no standard of care currently exists for recurrent cases. Glioma stem cells (GSCs), a rare and highly aggressive subpopulation of cells within these tumors, have recently emerged as drivers of tumor initiation and recurrence, and a growing body of evidence suggests that they must be completely eradicated to prevent relapse. Toward this goal, we have developed polyethylenimine-wrapped spherical nucleic acid nanoparticles (PEI–SNAs) targeting Gli1, a transcription factor within the Hedgehog signaling pathway that is crucial for the maintenance of GSCs. Here, we demonstrate that Gli1 PEI–SNAs bind scavenger receptors on GBM cells to undergo endocytosis in a caveolae/lipid raft/dynamin-dependent manner. They further achieve ∼30% silencing of tumor-promoting Hedgehog pathway genes and downstream target genes that promote the aggressive, chemoresistant phenotype of GBM. This produces a 30% decrease in proliferation that correlates with a robust onset of GBM cell senescence as well as an ∼60% decrease in metabolic activity with or without cotreatment with temozolomide (TMZ), the frontline chemotherapy for GBM. Most importantly, Gli1 PEI–SNAs impair the self-renewal capacity of GBM cells as indicated by a 30–40% reduction in the expression of stemness genes and further impair the formation of stem-like neurospheres. They also substantially improve neurosphere chemosensitivity as demonstrated by a 2-fold increase in the fraction of cells undergoing apoptosis in response to low doses of TMZ. These results underscore the potential for siRNA therapeutics targeting Gli1 to reduce GBM resistance to therapy and warrant further development of PEI–SNAs and Gli1-targeted therapies to alleviate drug resistance and recurrence for GBM patients.
中文翻译:

抗 Gli1 的聚乙烯亚胺球形核酸纳米粒子降低胶质母细胞瘤细胞的化疗耐药性和干性
胶质母细胞瘤 (GBM) 是成人中最常见和致命的原发性脑肿瘤,近 100% 的患者最终死于该疾病。患者的中位生存期为 15 个月,目前尚无针对复发病例的护理标准。神经胶质瘤干细胞(GSC)是肿瘤内一种罕见且高度侵袭性的细胞亚群,最近已成为肿瘤发生和复发的驱动因素,越来越多的证据表明必须完全根除它们以防止复发。为了实现这一目标,我们开发了针对 Gli1 的聚乙烯亚胺包裹的球形核酸纳米颗粒 (PEI-SNA),Gli1 是 Hedgehog 信号通路中的转录因子,对于 GSC 的维持至关重要。在这里,我们证明 Gli1 PEI-SNA 结合 GBM 细胞上的清道夫受体,以小凹/脂筏/动力依赖性方式进行内吞作用。他们进一步实现了促肿瘤 Hedgehog 通路基因和促进 GBM 侵袭性、化疗耐药表型的下游靶基因约 30% 的沉默。这会导致增殖减少 30%,与 GBM 细胞衰老的剧烈开始相关,并且无论是否使用替莫唑胺 (TMZ)(GBM 的一线化疗)联合治疗,代谢活性都会降低约 60%。最重要的是,Gli1 PEI-SNA 损害 GBM 细胞的自我更新能力,干细胞基因表达减少 30-40%,并进一步损害干细胞样神经球的形成。它们还显着改善了神经球的化学敏感性,低剂量 TMZ 导致发生凋亡的细胞比例增加了 2 倍,证明了这一点。这些结果强调了针对 Gli1 的 siRNA 疗法降低 GBM 对治疗的耐药性的潜力,并保证进一步开发 PEI-SNA 和 Gli1 靶向疗法以减轻 GBM 患者的耐药性和复发。
更新日期:2018-09-27
中文翻译:

抗 Gli1 的聚乙烯亚胺球形核酸纳米粒子降低胶质母细胞瘤细胞的化疗耐药性和干性
胶质母细胞瘤 (GBM) 是成人中最常见和致命的原发性脑肿瘤,近 100% 的患者最终死于该疾病。患者的中位生存期为 15 个月,目前尚无针对复发病例的护理标准。神经胶质瘤干细胞(GSC)是肿瘤内一种罕见且高度侵袭性的细胞亚群,最近已成为肿瘤发生和复发的驱动因素,越来越多的证据表明必须完全根除它们以防止复发。为了实现这一目标,我们开发了针对 Gli1 的聚乙烯亚胺包裹的球形核酸纳米颗粒 (PEI-SNA),Gli1 是 Hedgehog 信号通路中的转录因子,对于 GSC 的维持至关重要。在这里,我们证明 Gli1 PEI-SNA 结合 GBM 细胞上的清道夫受体,以小凹/脂筏/动力依赖性方式进行内吞作用。他们进一步实现了促肿瘤 Hedgehog 通路基因和促进 GBM 侵袭性、化疗耐药表型的下游靶基因约 30% 的沉默。这会导致增殖减少 30%,与 GBM 细胞衰老的剧烈开始相关,并且无论是否使用替莫唑胺 (TMZ)(GBM 的一线化疗)联合治疗,代谢活性都会降低约 60%。最重要的是,Gli1 PEI-SNA 损害 GBM 细胞的自我更新能力,干细胞基因表达减少 30-40%,并进一步损害干细胞样神经球的形成。它们还显着改善了神经球的化学敏感性,低剂量 TMZ 导致发生凋亡的细胞比例增加了 2 倍,证明了这一点。这些结果强调了针对 Gli1 的 siRNA 疗法降低 GBM 对治疗的耐药性的潜力,并保证进一步开发 PEI-SNA 和 Gli1 靶向疗法以减轻 GBM 患者的耐药性和复发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号