当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Suppression of Aggregation of Therapeutic Monoclonal Antibodies during Storage by Removal of Aggregation Precursors Using a Specific Adsorbent of Non-Native IgG Conformers
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-09-28 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00360 Yukako Senga 1 , Shinya Honda 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2018-09-28 00:00:00 , DOI: 10.1021/acs.bioconjchem.8b00360 Yukako Senga 1 , Shinya Honda 1
Affiliation
|
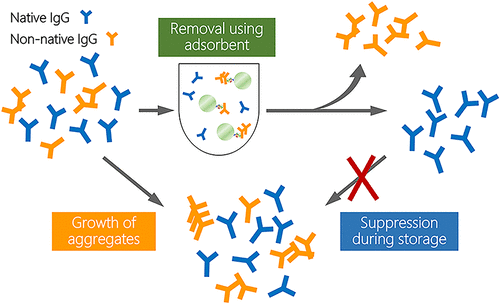
The quality of preparations of therapeutic IgG molecules, widely used for the treatment of various diseases, should be maintained during storage and administration. Nevertheless, recent studies demonstrate that IgG aggregation is one of the most critical immunogenicity risk factors that compromises safety and efficacy of therapeutic IgG molecules in the clinical setting. During the IgG manufacturing process, 0.22-μm membrane filters are commonly used to remove aggregates. However, particles with a diameter below 0.22 μm (small aggregates) are not removed from the final product. The residual species may grow into large aggregates during the storage period. In the current study, we devised a strategy to suppress IgG aggregate growth by removing aggregation precursors using the artificial protein AF.2A1. This protein efficiently binds the Fc region of non-native IgG conformers generated under chemical and physical stresses. Magnetic beads conjugated with AF.2A1 were used to remove non-native monomers and aggregates from solutions of native IgG and from native IgG solutions spiked with stressed IgG. The time-dependent growth of aggregates after the removal treatment was monitored. The removal of aggregation precursors, i.e., non-native monomers and nanometer aggregates (<100 nm), suppressed the aggregate growth. The presented findings demonstrate that a removal treatment with a specific adsorbent of non-native IgG conformers enables long-term stable storage of therapeutic IgG molecules and will facilitate mitigation of the immunogenicity of IgG preparations.
中文翻译:

通过使用非天然IgG构型异构体的特异性吸附剂去除聚集前体来抑制治疗性单克隆抗体的聚集
在储存和给药过程中应保持被广泛用于治疗各种疾病的治疗性IgG分子制剂的质量。尽管如此,最近的研究表明,IgG聚集是最关键的免疫原性危险因素之一,在临床环境中会损害治疗性IgG分子的安全性和有效性。在IgG生产过程中,通常使用0.22μm的膜滤器去除聚集体。但是,没有从最终产品中除去直径小于0.22μm的颗粒(小聚集体)。在储存期间,残留物可能会长成大的聚集体。在当前的研究中,我们设计了一种策略,可以通过使用人工蛋白AF.2A1去除聚集前体来抑制IgG聚集生长。该蛋白质有效结合在化学和物理压力下产生的非天然IgG构象异构体的Fc区。使用与AF.2A1共轭的磁珠从天然IgG溶液和加应力IgG的天然IgG溶液中去除非天然单体和聚集体。监测去除处理后聚集体随时间的增长。去除聚集体前体,即非天然单体和纳米聚集体(<100 nm),抑制了聚集体的生长。提出的发现表明,用非天然IgG构象异构体的特异性吸附剂进行的去除处理能够长期稳定地保存治疗性IgG分子,并有助于减轻IgG制剂的免疫原性。使用与AF.2A1共轭的磁珠从天然IgG溶液和加应力IgG的天然IgG溶液中去除非天然单体和聚集体。监测去除处理后聚集体随时间的增长。去除聚集体前体,即非天然单体和纳米聚集体(<100 nm),抑制了聚集体的生长。提出的发现表明,用非天然IgG构象异构体的特异性吸附剂进行的去除处理能够长期稳定地保存治疗性IgG分子,并有助于减轻IgG制剂的免疫原性。使用与AF.2A1共轭的磁珠从天然IgG溶液和加应力IgG的天然IgG溶液中去除非天然单体和聚集体。监测去除处理后聚集体随时间的增长。去除聚集体前体,即非天然单体和纳米聚集体(<100 nm),抑制了聚集体的生长。提出的发现表明,用非天然IgG构象异构体的特异性吸附剂进行的去除处理能够长期稳定地保存治疗性IgG分子,并有助于减轻IgG制剂的免疫原性。监测去除处理后聚集体随时间的增长。去除聚集体前体,即非天然单体和纳米聚集体(<100 nm),抑制了聚集体的生长。提出的发现表明,用非天然IgG构象异构体的特异性吸附剂进行的去除处理能够长期稳定地保存治疗性IgG分子,并有助于减轻IgG制剂的免疫原性。监测去除处理后聚集体随时间的增长。去除聚集体前体,即非天然单体和纳米聚集体(<100 nm),抑制了聚集体的生长。提出的发现表明,用非天然IgG构象异构体的特异性吸附剂进行的去除处理能够长期稳定地保存治疗性IgG分子,并有助于减轻IgG制剂的免疫原性。
更新日期:2018-09-28
中文翻译:

通过使用非天然IgG构型异构体的特异性吸附剂去除聚集前体来抑制治疗性单克隆抗体的聚集
在储存和给药过程中应保持被广泛用于治疗各种疾病的治疗性IgG分子制剂的质量。尽管如此,最近的研究表明,IgG聚集是最关键的免疫原性危险因素之一,在临床环境中会损害治疗性IgG分子的安全性和有效性。在IgG生产过程中,通常使用0.22μm的膜滤器去除聚集体。但是,没有从最终产品中除去直径小于0.22μm的颗粒(小聚集体)。在储存期间,残留物可能会长成大的聚集体。在当前的研究中,我们设计了一种策略,可以通过使用人工蛋白AF.2A1去除聚集前体来抑制IgG聚集生长。该蛋白质有效结合在化学和物理压力下产生的非天然IgG构象异构体的Fc区。使用与AF.2A1共轭的磁珠从天然IgG溶液和加应力IgG的天然IgG溶液中去除非天然单体和聚集体。监测去除处理后聚集体随时间的增长。去除聚集体前体,即非天然单体和纳米聚集体(<100 nm),抑制了聚集体的生长。提出的发现表明,用非天然IgG构象异构体的特异性吸附剂进行的去除处理能够长期稳定地保存治疗性IgG分子,并有助于减轻IgG制剂的免疫原性。使用与AF.2A1共轭的磁珠从天然IgG溶液和加应力IgG的天然IgG溶液中去除非天然单体和聚集体。监测去除处理后聚集体随时间的增长。去除聚集体前体,即非天然单体和纳米聚集体(<100 nm),抑制了聚集体的生长。提出的发现表明,用非天然IgG构象异构体的特异性吸附剂进行的去除处理能够长期稳定地保存治疗性IgG分子,并有助于减轻IgG制剂的免疫原性。使用与AF.2A1共轭的磁珠从天然IgG溶液和加应力IgG的天然IgG溶液中去除非天然单体和聚集体。监测去除处理后聚集体随时间的增长。去除聚集体前体,即非天然单体和纳米聚集体(<100 nm),抑制了聚集体的生长。提出的发现表明,用非天然IgG构象异构体的特异性吸附剂进行的去除处理能够长期稳定地保存治疗性IgG分子,并有助于减轻IgG制剂的免疫原性。监测去除处理后聚集体随时间的增长。去除聚集体前体,即非天然单体和纳米聚集体(<100 nm),抑制了聚集体的生长。提出的发现表明,用非天然IgG构象异构体的特异性吸附剂进行的去除处理能够长期稳定地保存治疗性IgG分子,并有助于减轻IgG制剂的免疫原性。监测去除处理后聚集体随时间的增长。去除聚集体前体,即非天然单体和纳米聚集体(<100 nm),抑制了聚集体的生长。提出的发现表明,用非天然IgG构象异构体的特异性吸附剂进行的去除处理能够长期稳定地保存治疗性IgG分子,并有助于减轻IgG制剂的免疫原性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号