当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Insight into Gate-Opening Adsorption Mechanism and Sigmoidal Adsorption Isotherm into Porous Coordination Polymer
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-09-28 , DOI: 10.1021/jacs.8b09358 Jia-Jia Zheng 1, 2 , Shinpei Kusaka 1 , Ryotaro Matsuda 1, 3 , Susumu Kitagawa 1 , Shigeyoshi Sakaki 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-09-28 , DOI: 10.1021/jacs.8b09358 Jia-Jia Zheng 1, 2 , Shinpei Kusaka 1 , Ryotaro Matsuda 1, 3 , Susumu Kitagawa 1 , Shigeyoshi Sakaki 2
Affiliation
|
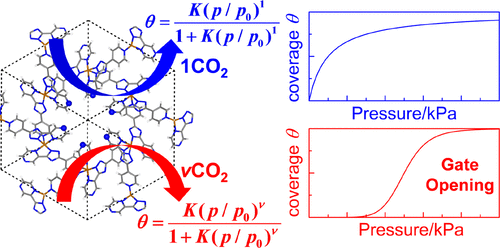
The gate-opening adsorption mechanism and sigmoidal adsorption isotherm were theoretically investigated taking CO2 adsorption into porous coordination polymers, [Fe(ppt)2] n (PCP-N, Hppt = 3-(2-pyrazinyl)-5-(4-pyridyl)-1,2,4-triazole) and [Fe(dpt)2] n (PCP-C, Hdpt = 3-(2-pyridinyl)-5-(4-pyridyl)-1,2,4-triazole) as examples, where the hybrid method consisting of dispersion-corrected DFT for infinite PCP and a post-Hartree-Fock (SCS-MP2 and CCSD(T)) method for the cluster model was employed. PCP-N has site I (one-dimensional channel), site II (small aperture to site I), and site III (small pore) useful for CO2 adsorption. CO2 adsorption at site I occurs in a one by one manner with a Langmuir adsorption isotherm. CO2 adsorption at sites II and III occurs through a gate-opening adsorption mechanism, because the crystal deformation energy ( EDEF) at these sites is induced largely by the first CO2 adsorption but induced much less by the subsequent CO2 adsorption. Interestingly, nine CO2 molecules are adsorbed simultaneously at these sites because a large EDEF cannot be overcome by adsorption of one CO2 molecule but can be by simultaneous adsorption of nine CO2 molecules. For such CO2 adsorption, the Langmuir-Freundlich sigmoidal adsorption isotherm was derived from the equilibrium equation for CO2 adsorption. A very complicated CO2 adsorption isotherm, experimentally observed, is reproduced by combination of the Langmuir and Langmuir-Freundlich adsorption isotherms. In PCP-C, CO2 adsorption occurs only at site I with the Langmuir adsorption isotherm. Sites II and III of PCP-C cannot be used for CO2 adsorption because a very large EDEF cannot be overcome by simultaneous adsorption of nine CO2 molecules. Factors necessary for gate-opening adsorption mechanism are discussed on the basis of differences between PCP-N and PCP-C.
中文翻译:

多孔配位聚合物的开门吸附机制和S形吸附等温线的理论洞察
将CO2吸附到多孔配位聚合物[Fe(ppt)2] n (PCP-N, Hppt = 3-(2-pyrazizyl)-5-(4-pyridyl) )-1,2,4-三唑) 和 [Fe(dpt)2] n (PCP-C, Hdpt = 3-(2-吡啶基)-5-(4-吡啶基)-1,2,4-三唑)例如,其中采用了由用于无限 PCP 的色散校正 DFT 和用于聚类模型的后 Hartree-Fock(SCS-MP2 和 CCSD(T))方法组成的混合方法。PCP-N 具有可用于 CO2 吸附的位点 I(一维通道)、位点 II(到位点 I 的小孔径)和位点 III(小孔)。位点 I 处的 CO2 吸附以朗缪尔吸附等温线的一种方式发生。位点 II 和 III 处的 CO2 吸附通过开门吸附机制发生,因为这些位点的晶体变形能 (EDEF) 主要是由第一次 CO2 吸附引起的,但由随后的 CO2 吸附引起的要少得多。有趣的是,在这些位置同时吸附了 9 个 CO2 分子,因为一个大的 EDEF 不能通过吸附一个 CO2 分子来克服,但可以通过同时吸附 9 个 CO2 分子来克服。对于这种 CO2 吸附,Langmuir-Freundlich Sigmoidal 吸附等温线是从 CO2 吸附的平衡方程导出的。实验观察到的非常复杂的 CO2 吸附等温线通过 Langmuir 和 Langmuir-Freundlich 吸附等温线的组合再现。在 PCP-C 中,CO2 吸附仅发生在具有朗缪尔吸附等温线的位点 I。PCP-C 的位点 II 和 III 不能用于 CO2 吸附,因为同时吸附九个 CO2 分子无法克服非常大的 EDEF。根据PCP-N和PCP-C之间的差异讨论了开闸吸附机制所需的因素。
更新日期:2018-09-28
中文翻译:

多孔配位聚合物的开门吸附机制和S形吸附等温线的理论洞察
将CO2吸附到多孔配位聚合物[Fe(ppt)2] n (PCP-N, Hppt = 3-(2-pyrazizyl)-5-(4-pyridyl) )-1,2,4-三唑) 和 [Fe(dpt)2] n (PCP-C, Hdpt = 3-(2-吡啶基)-5-(4-吡啶基)-1,2,4-三唑)例如,其中采用了由用于无限 PCP 的色散校正 DFT 和用于聚类模型的后 Hartree-Fock(SCS-MP2 和 CCSD(T))方法组成的混合方法。PCP-N 具有可用于 CO2 吸附的位点 I(一维通道)、位点 II(到位点 I 的小孔径)和位点 III(小孔)。位点 I 处的 CO2 吸附以朗缪尔吸附等温线的一种方式发生。位点 II 和 III 处的 CO2 吸附通过开门吸附机制发生,因为这些位点的晶体变形能 (EDEF) 主要是由第一次 CO2 吸附引起的,但由随后的 CO2 吸附引起的要少得多。有趣的是,在这些位置同时吸附了 9 个 CO2 分子,因为一个大的 EDEF 不能通过吸附一个 CO2 分子来克服,但可以通过同时吸附 9 个 CO2 分子来克服。对于这种 CO2 吸附,Langmuir-Freundlich Sigmoidal 吸附等温线是从 CO2 吸附的平衡方程导出的。实验观察到的非常复杂的 CO2 吸附等温线通过 Langmuir 和 Langmuir-Freundlich 吸附等温线的组合再现。在 PCP-C 中,CO2 吸附仅发生在具有朗缪尔吸附等温线的位点 I。PCP-C 的位点 II 和 III 不能用于 CO2 吸附,因为同时吸附九个 CO2 分子无法克服非常大的 EDEF。根据PCP-N和PCP-C之间的差异讨论了开闸吸附机制所需的因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号