Nature Reviews Chemistry ( IF 38.1 ) Pub Date : 2018-09-27 , DOI: 10.1038/s41570-018-0041-7 Nicolai Lehnert , Hai T. Dong , Jill B. Harland , Andrew P. Hunt , Corey J. White
|
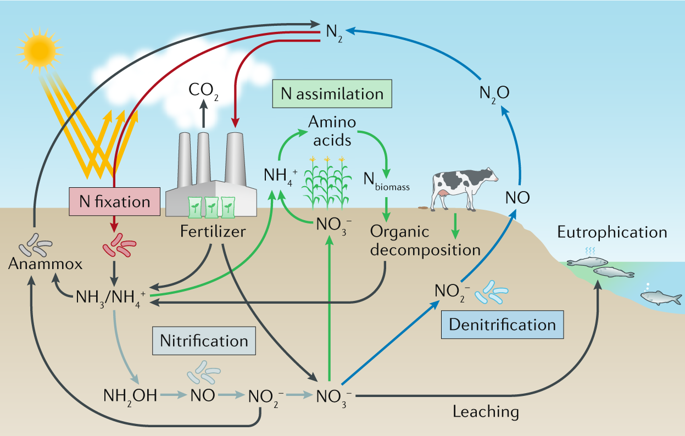
The nitrogen cycle is one of the most important biogeochemical cycles on Earth because nitrogen is an essential nutrient for all life forms. To supplement natural nitrogen fixation, farmers add large amounts of nitrogen-containing fertilizer to their soils such that nitrogen never becomes a limiting nutrient for plant growth. However, of the nitrogen added to fields — most of which is in the form of NH3 and NO3− — only 30–50% is taken up by plants, while the remainder is metabolized by soil microorganisms in processes with detrimental environmental impacts. The first of these processes, that is, nitrification, refers to the biological oxidation of NH3 to NO2− and NO3−, which have low retention in soil and pollute waterways, leading to downstream eutrophication and ultimately ‘dead zones’ (low oxygen zones) in coastal waters, for example, the Gulf of Mexico. In a second process, namely, denitrification, NO3− and NO2− undergo stepwise reduction to N2O and N2. Substantial amounts of the N2O produced in this process escape into the atmosphere, contributing to climate change and ozone destruction. Recent results suggest that nitrification also affords N2O. This Review describes the enzymes involved in NH3 oxidation and N2O production and degradation in the nitrogen cycle. We pay particular attention to the active site structures, the associated coordination chemistry that enables the chemical transformations and the reaction mechanisms.
中文翻译:

反向固氮
氮循环是地球上最重要的生物地球化学循环之一,因为氮是所有生命形式的必需营养素。为了补充自然固氮作用,农民向土壤中添加了大量的含氮肥料,以使氮永远不会成为植物生长的限制性养分。然而,添加到字段中的氮-其中大部分是在NH形式3和NO 3 - -只有30%-50%是采取了由植物,而其余部分是通过土壤中的微生物与不利的环境影响的过程代谢。第一这些过程,即,硝化,指的是NH的生物氧化3为NO 2 -和NO 3 -,它们在土壤和污染水道中的保留率低,导致下游富营养化,并最终导致沿海水域(例如墨西哥湾)的“死区”(低氧区)。在第二种方法中,即,脱硝,NO 3 -和NO 2 -经受逐步还原成N 2 O和N 2。在此过程中产生的大量N 2 O逸散到大气中,导致气候变化和臭氧破坏。最近的研究结果表明,硝化也提供Ñ 2 O.本回顾描述涉及NH酶3氧化和N 2在氮循环中O的产生和降解。我们特别注意活性位点结构,相关的配位化学,以实现化学转化和反应机理。









































 京公网安备 11010802027423号
京公网安备 11010802027423号